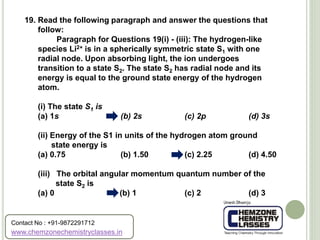
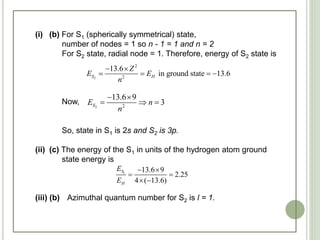
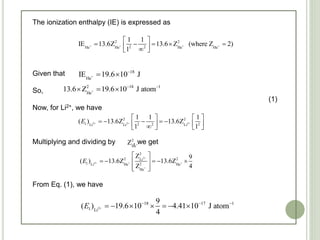
The document contains a series of multiple-choice questions related to chemistry concepts, particularly focusing on quantum mechanics, atomic structure, and the interactions of light with matter. Various calculations involving wavelengths, energy levels, and the behavior of electrons under different conditions are presented as part of the problem set. Additionally, the document references certain physical constants and principles, such as Heisenberg's uncertainty principle and Bohr's model of hydrogen, as they relate to the questions provided.












![(a) Mn3+
(b) Fe3+
(c) Co3+
(d) Ni3+
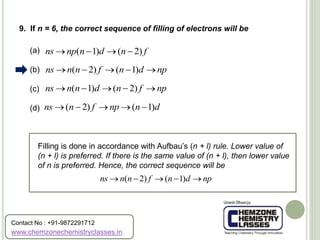
10. Which one of the following ions has electronic configuration
[Ar] 3d 6 ?
The atomic number of Co is 27. Now, for Co3+ (24),
the electronic configuration is
1s2 2s2 2p6 3s2 3p6 4s0 3d6 = [Ar]3d6
www.chemzonechemistryclasses.in
Contact No : +91-9872291712](https://image.slidesharecdn.com/atomicstructure-161115194139/85/Atomic-structure-Multiple-Choice-Questions-For-IIT-JEE-NEET-SAT-KVPY-15-320.jpg)