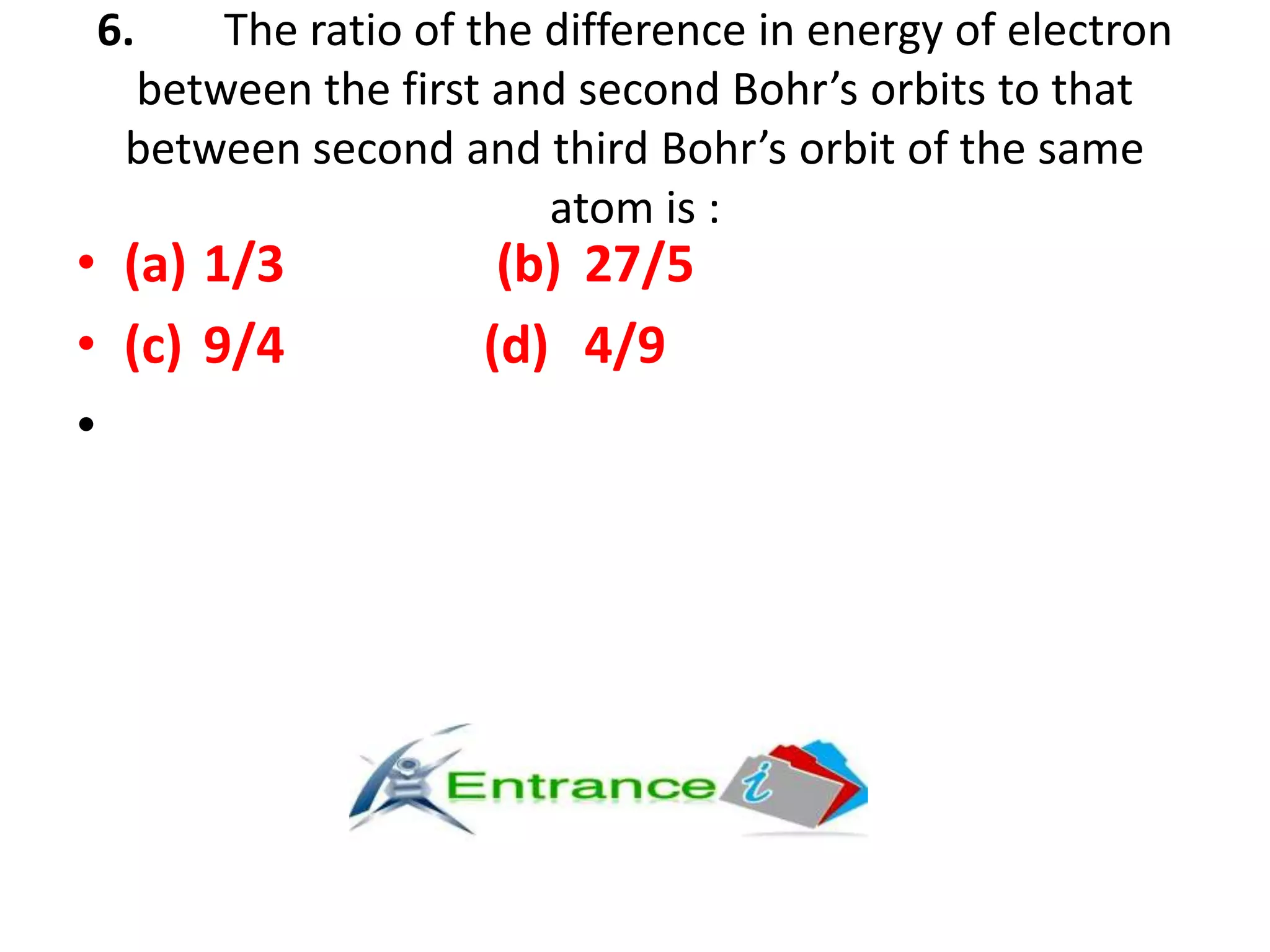
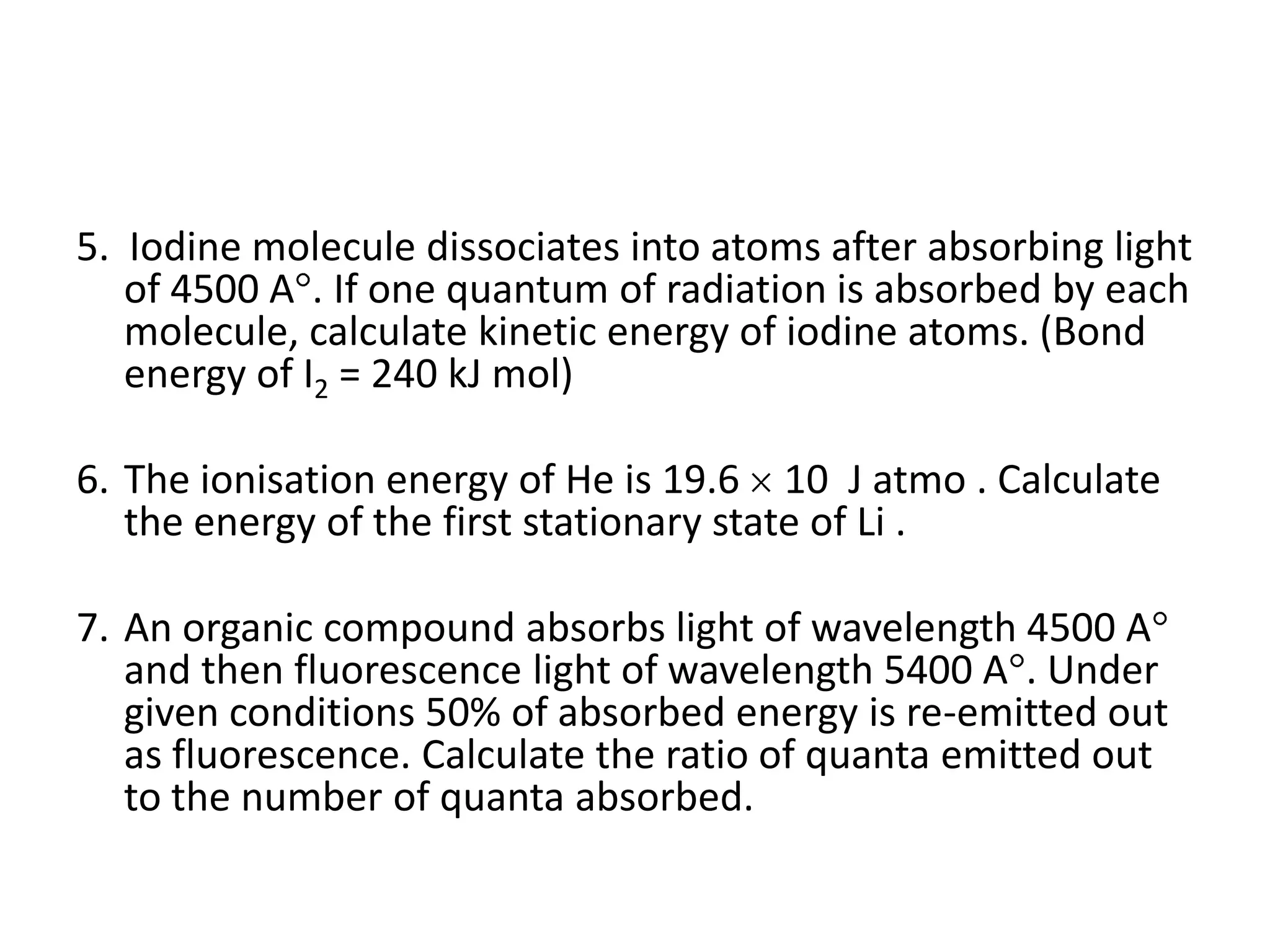
The document presents a series of questions and calculations related to atomic physics, focusing on concepts like ionization energies, spectral lines, and quantum mechanics within hydrogen and hydrogen-like ions. It includes multiple-choice questions and subjective problems requiring calculations related to photon energy, electron transitions, and atomic orbitals. The content targets a deeper understanding of atomic structure and behavior as described by Bohr's model and other quantum theories.