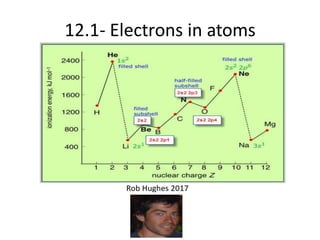
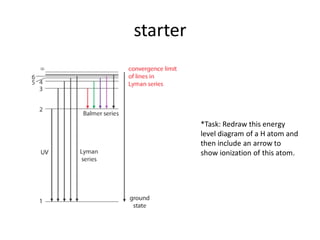
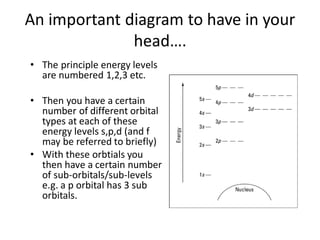
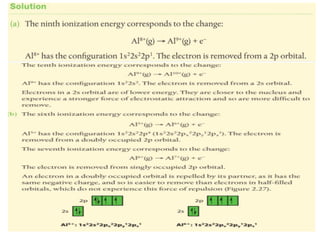
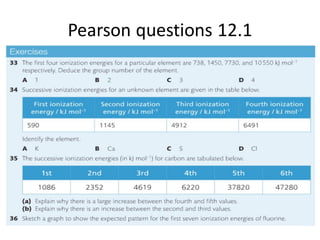
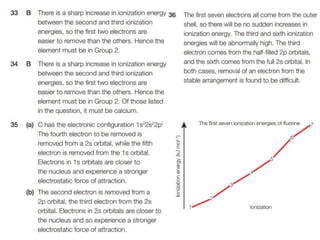
The document outlines lessons on atomic structure, focusing on ionization energy and its implications for atomic models. It includes concepts like isotopes, energy levels, and trends in ionization energy across periods in the periodic table. Additionally, it provides review questions and resources for further study, along with links to quizzes and video explanations.