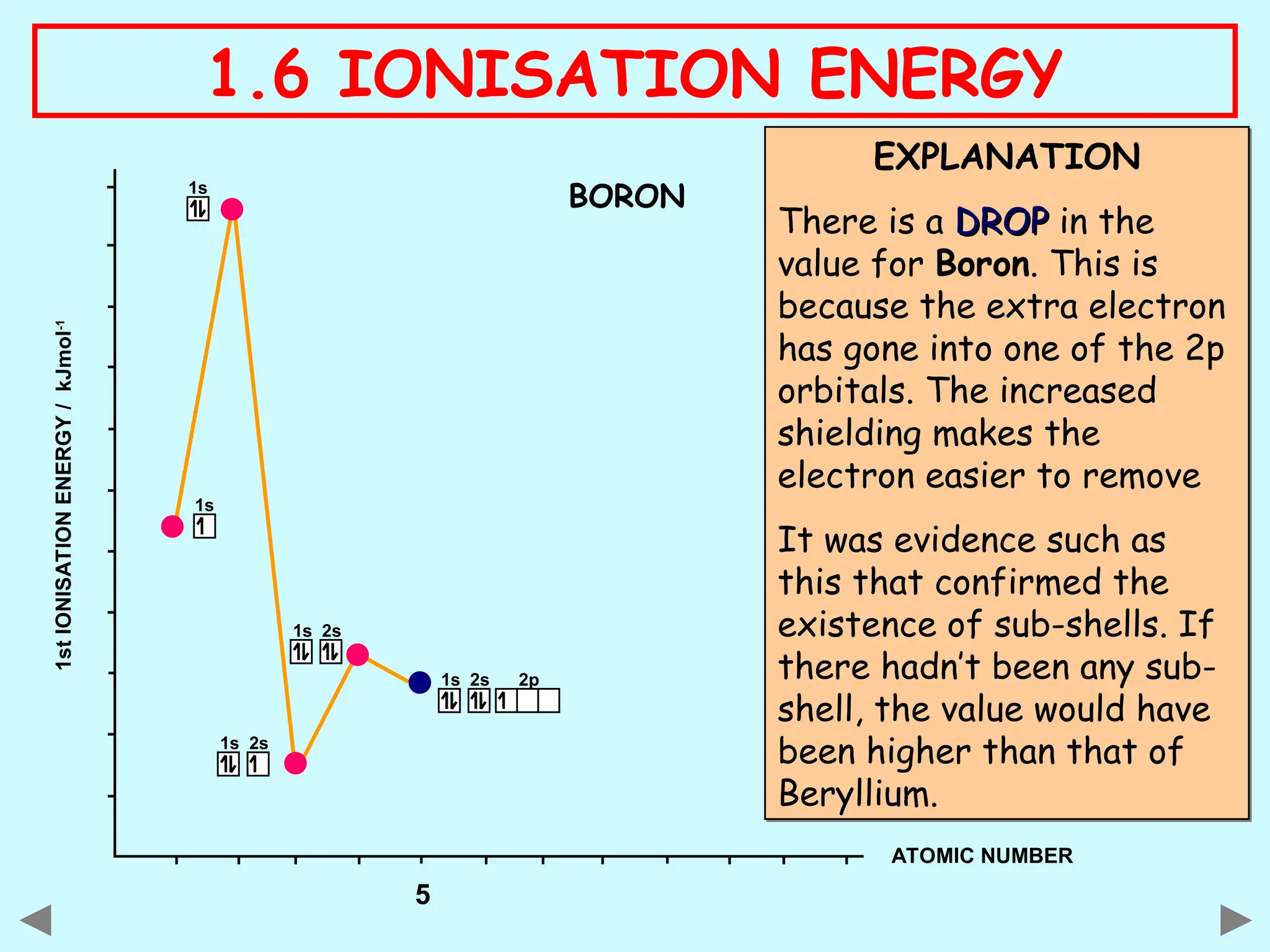
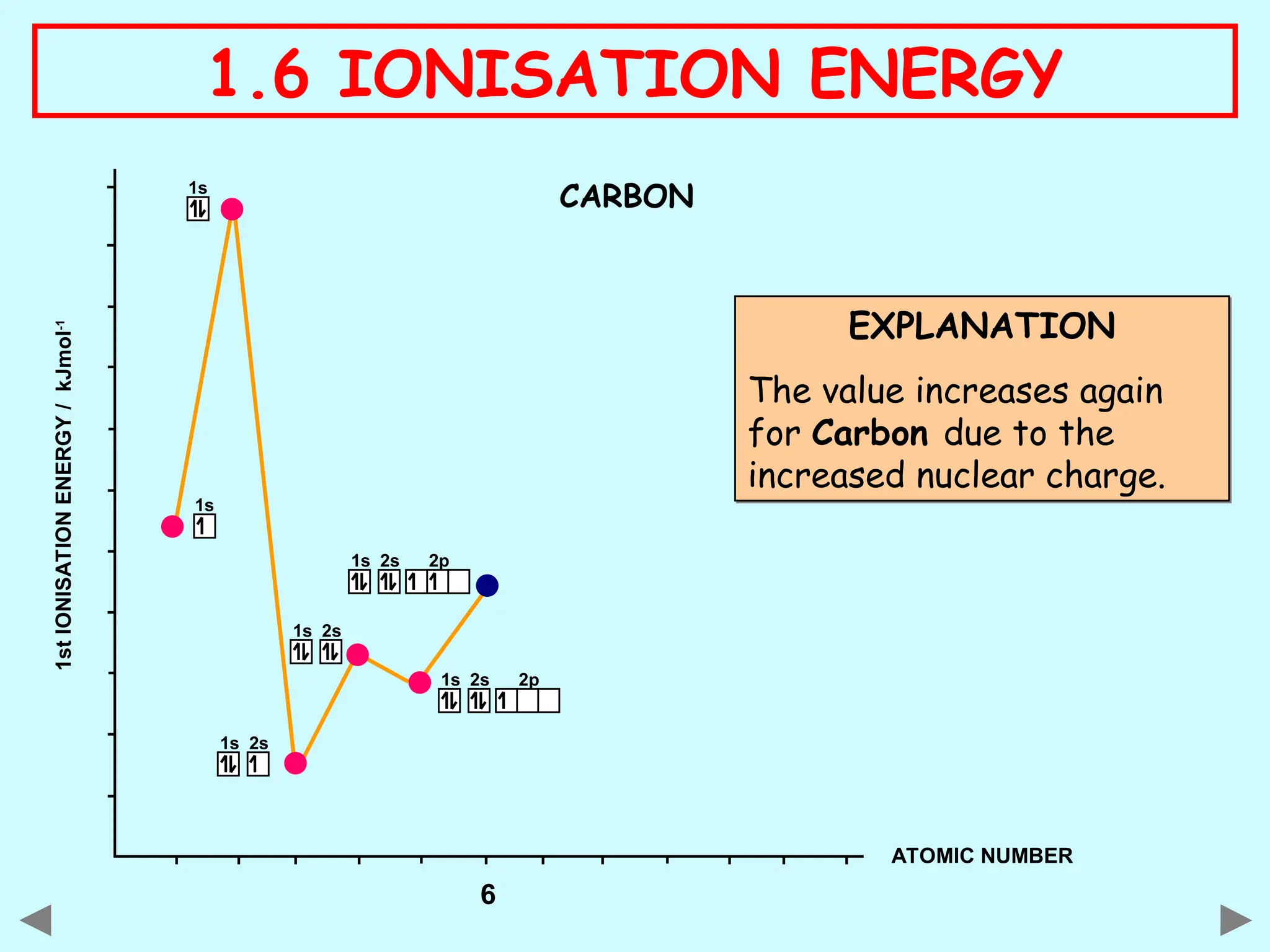
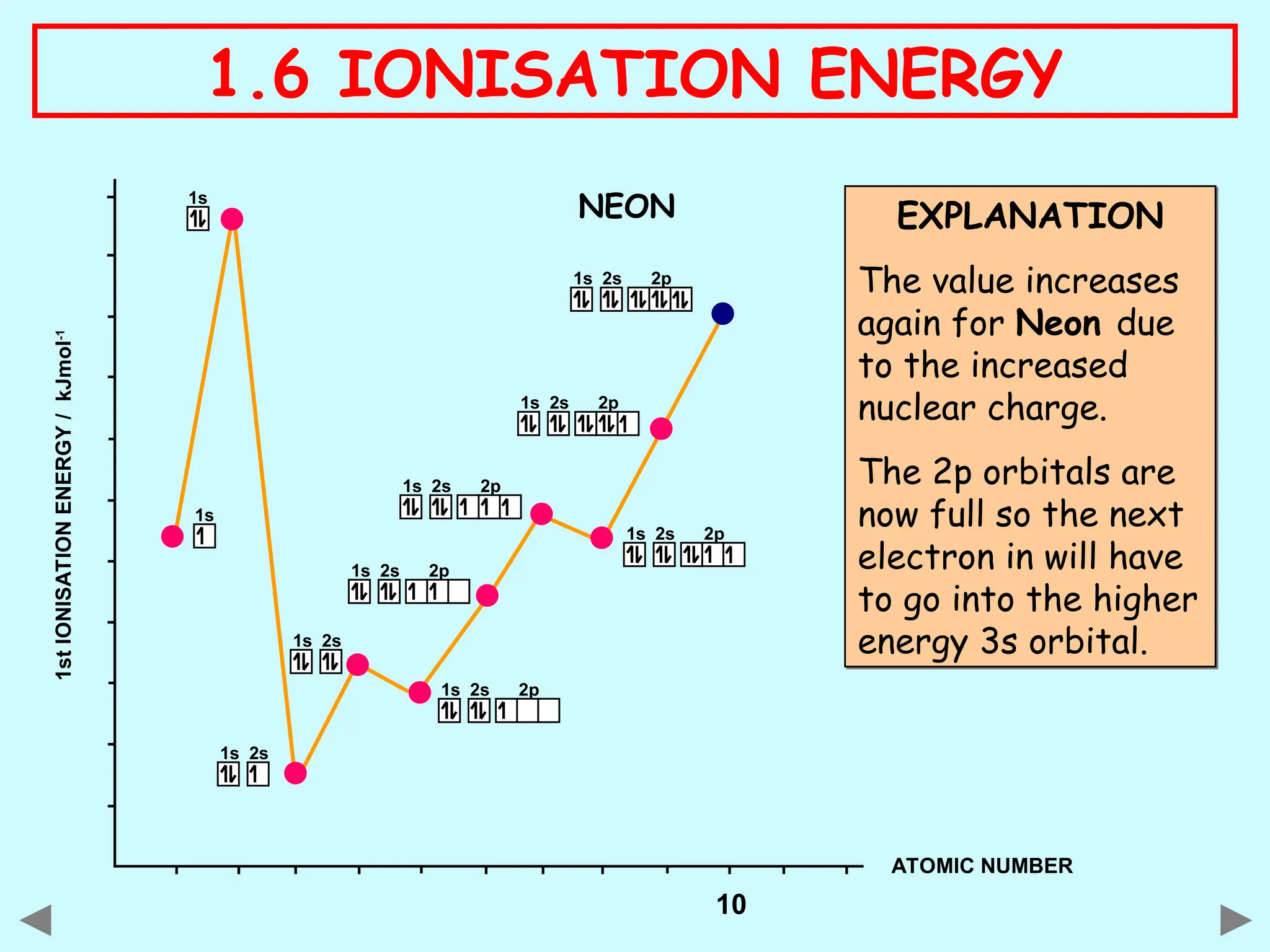
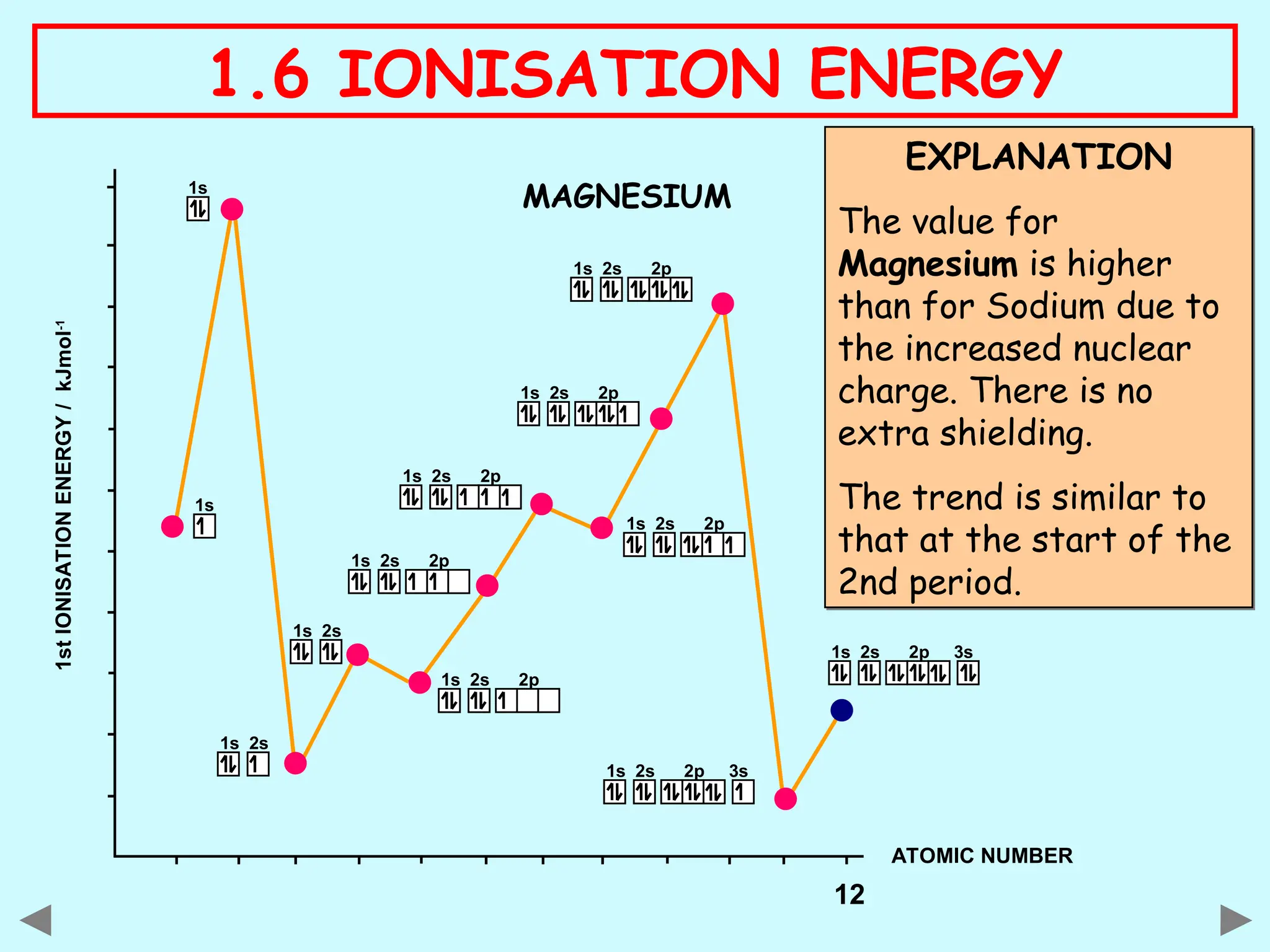
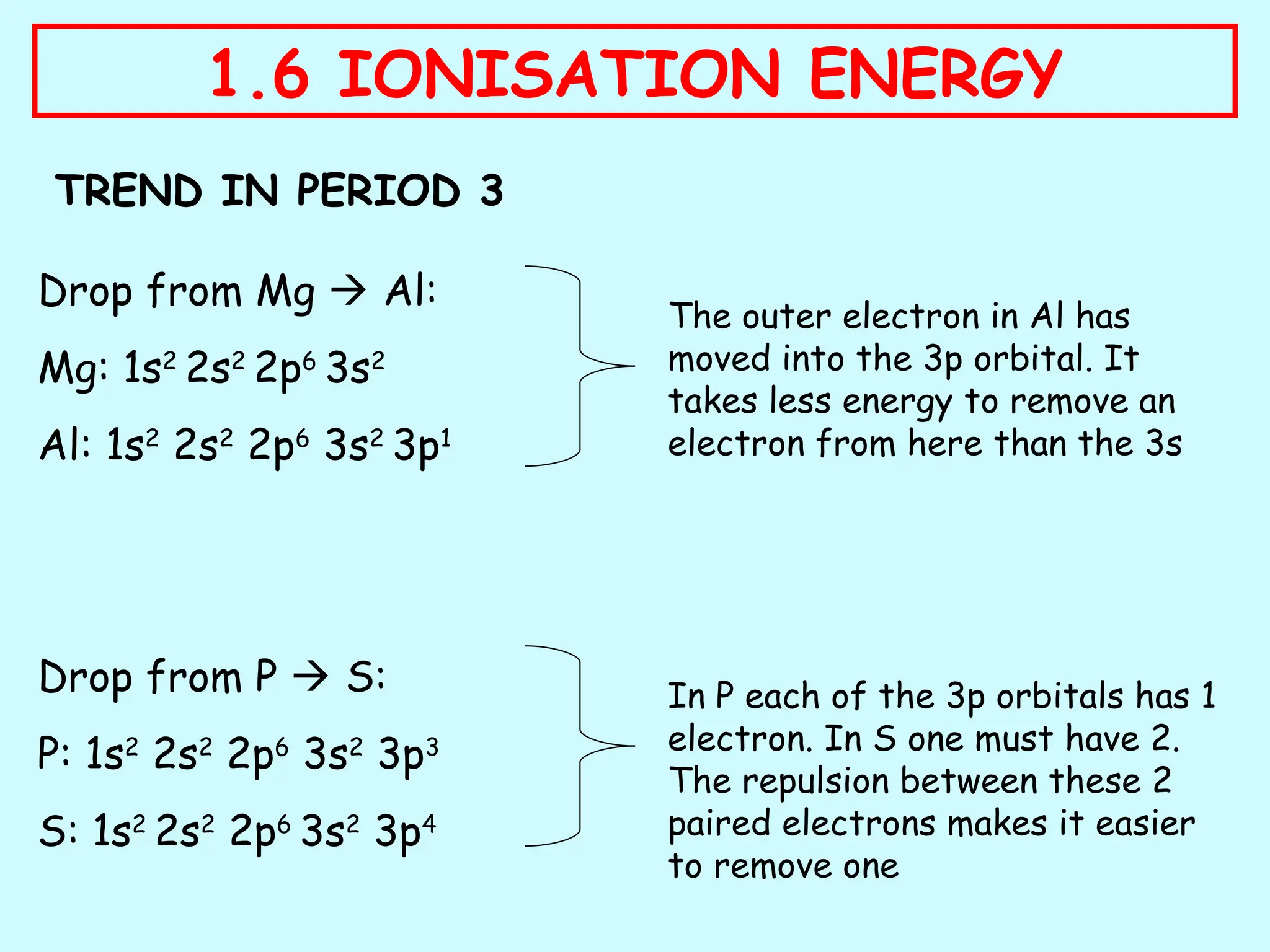
The document discusses ionisation energy, defining it as the energy required to remove one electron from gaseous atoms. It explains trends in ionisation energy across period 3 and down group 2, emphasizing factors such as atomic radius, nuclear charge, and electron shielding that affect these energies. The document provides detailed explanations of successive ionisation energies for various elements and indicates that ionisation energy generally increases across a period and decreases down a group due to electron configurations and shielding effects.