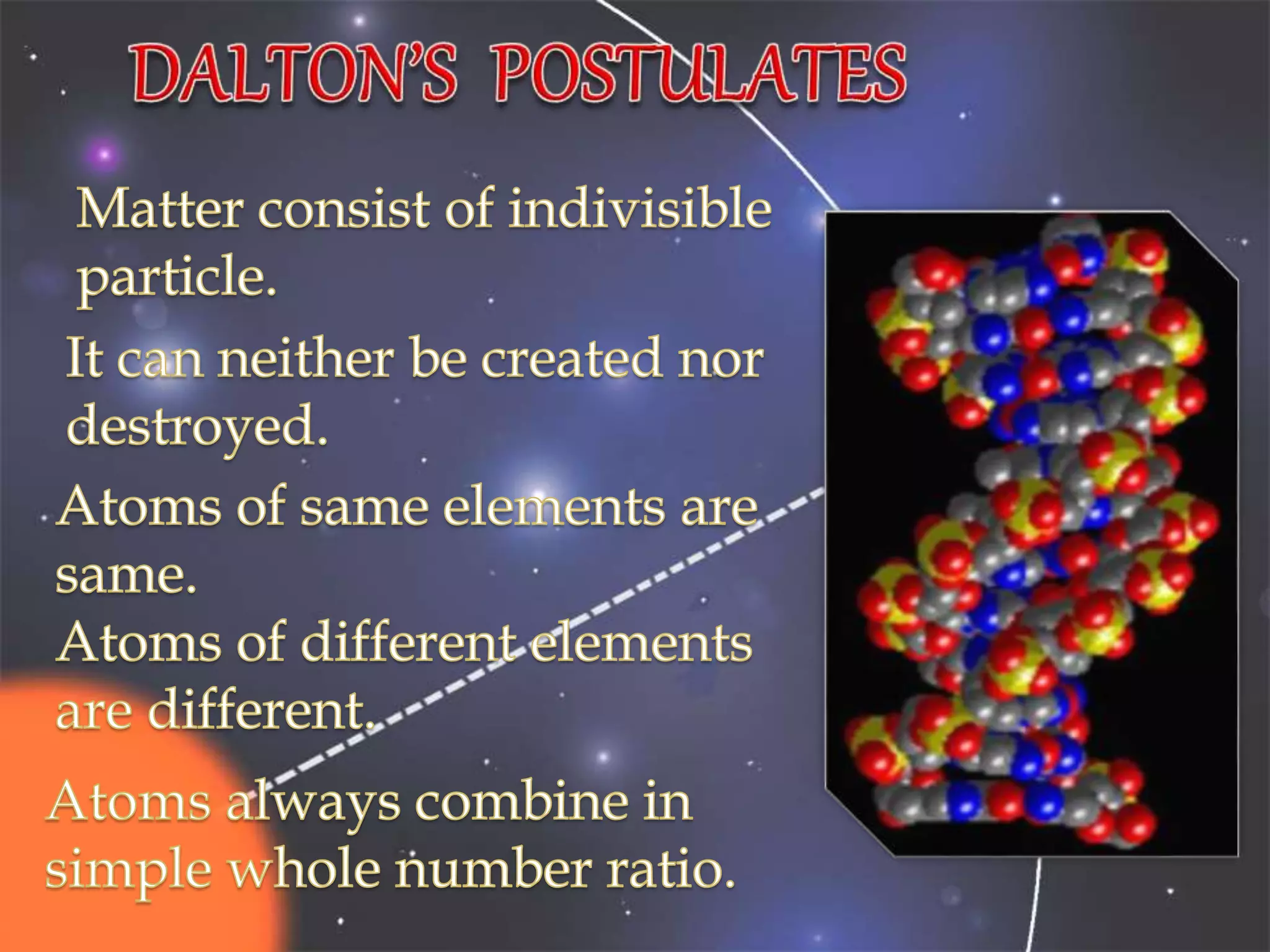
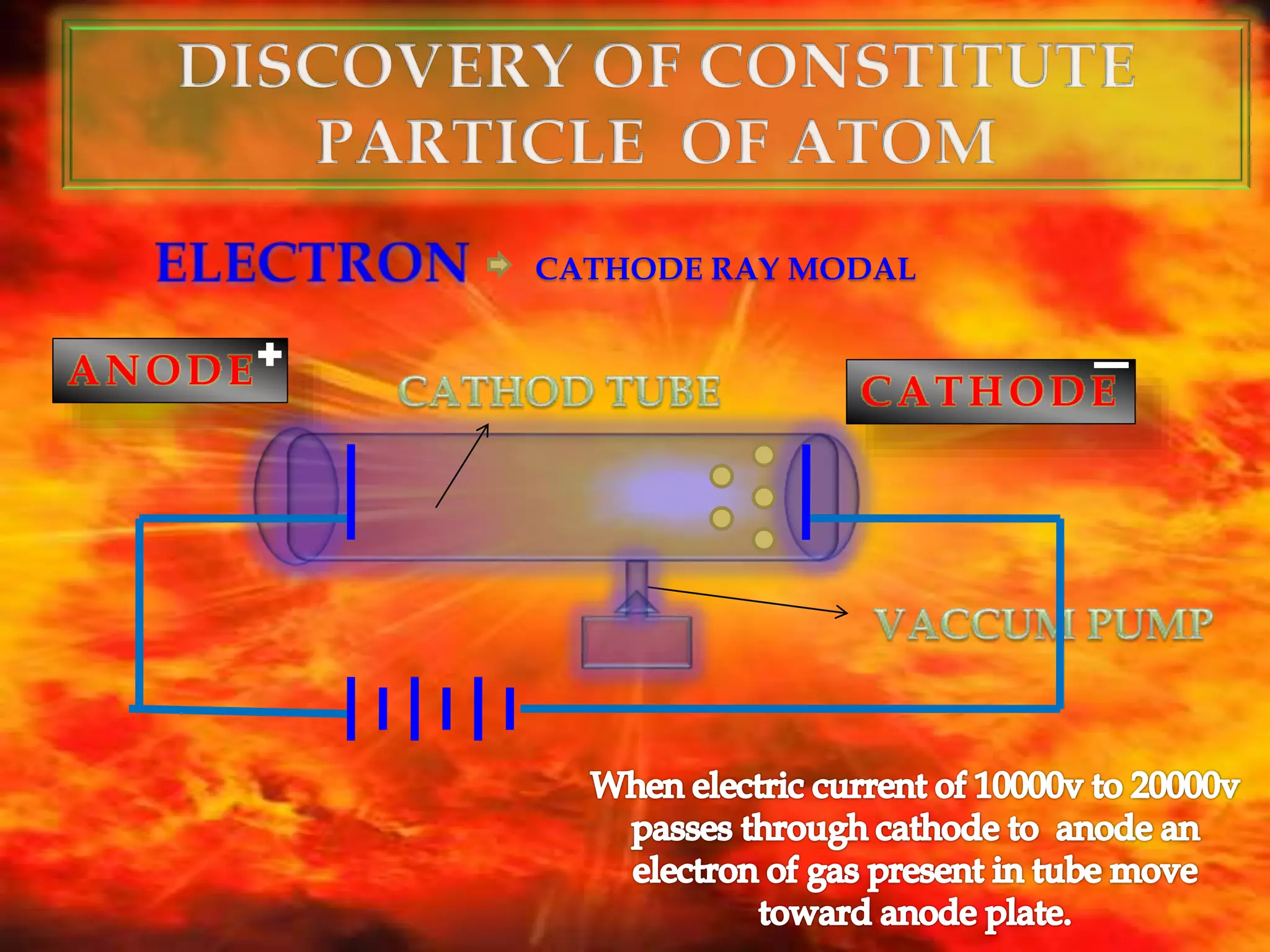
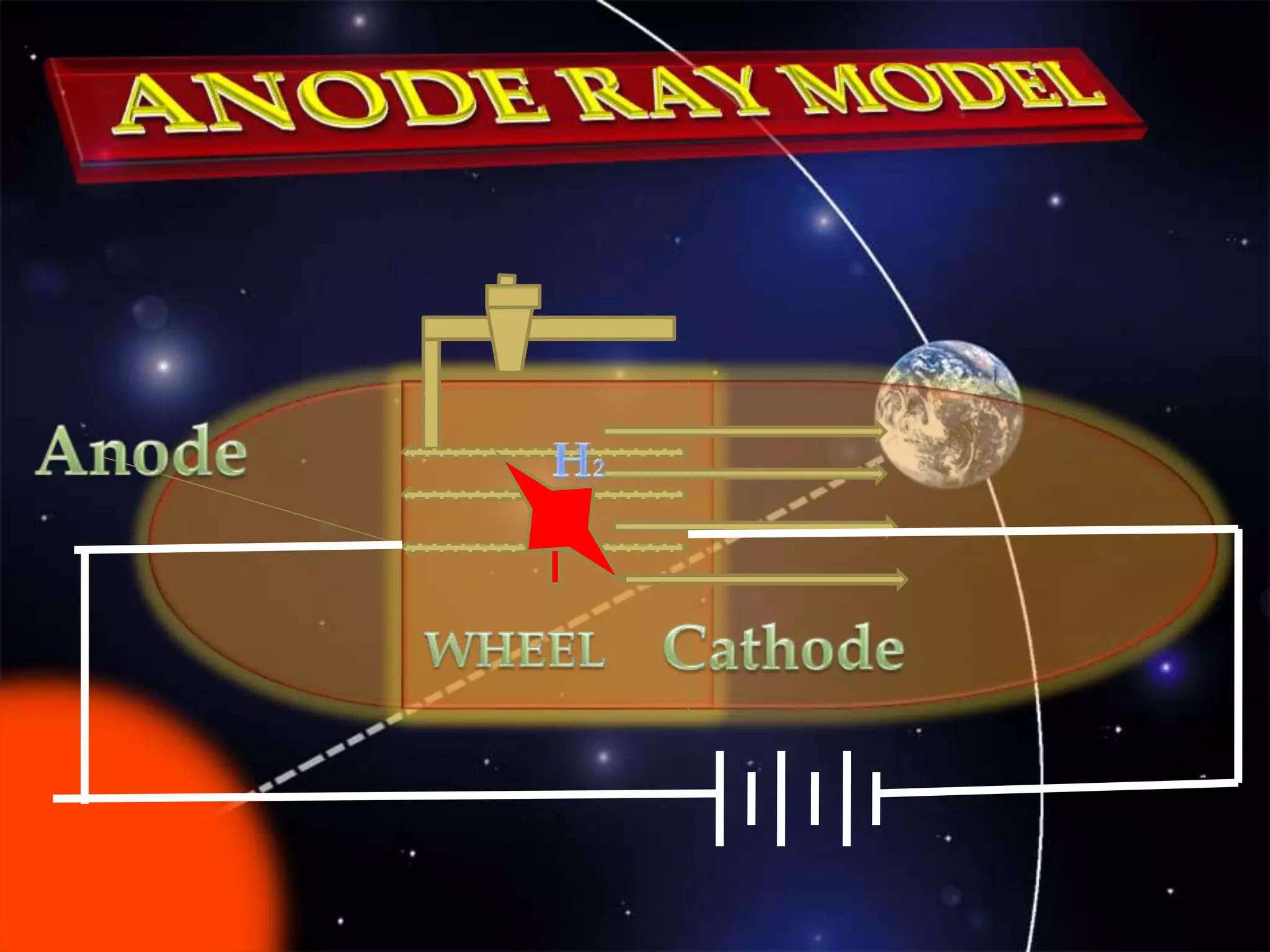
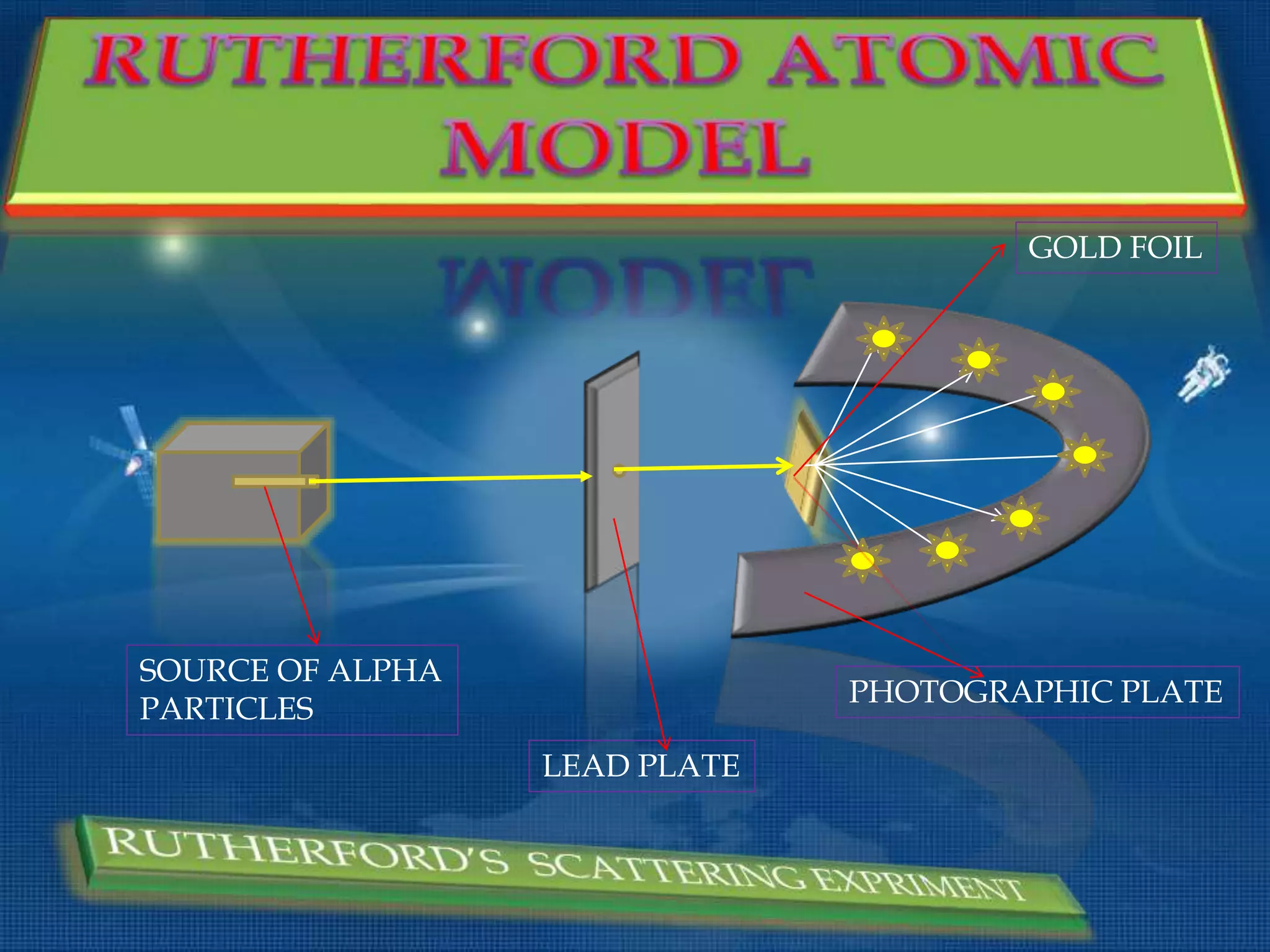
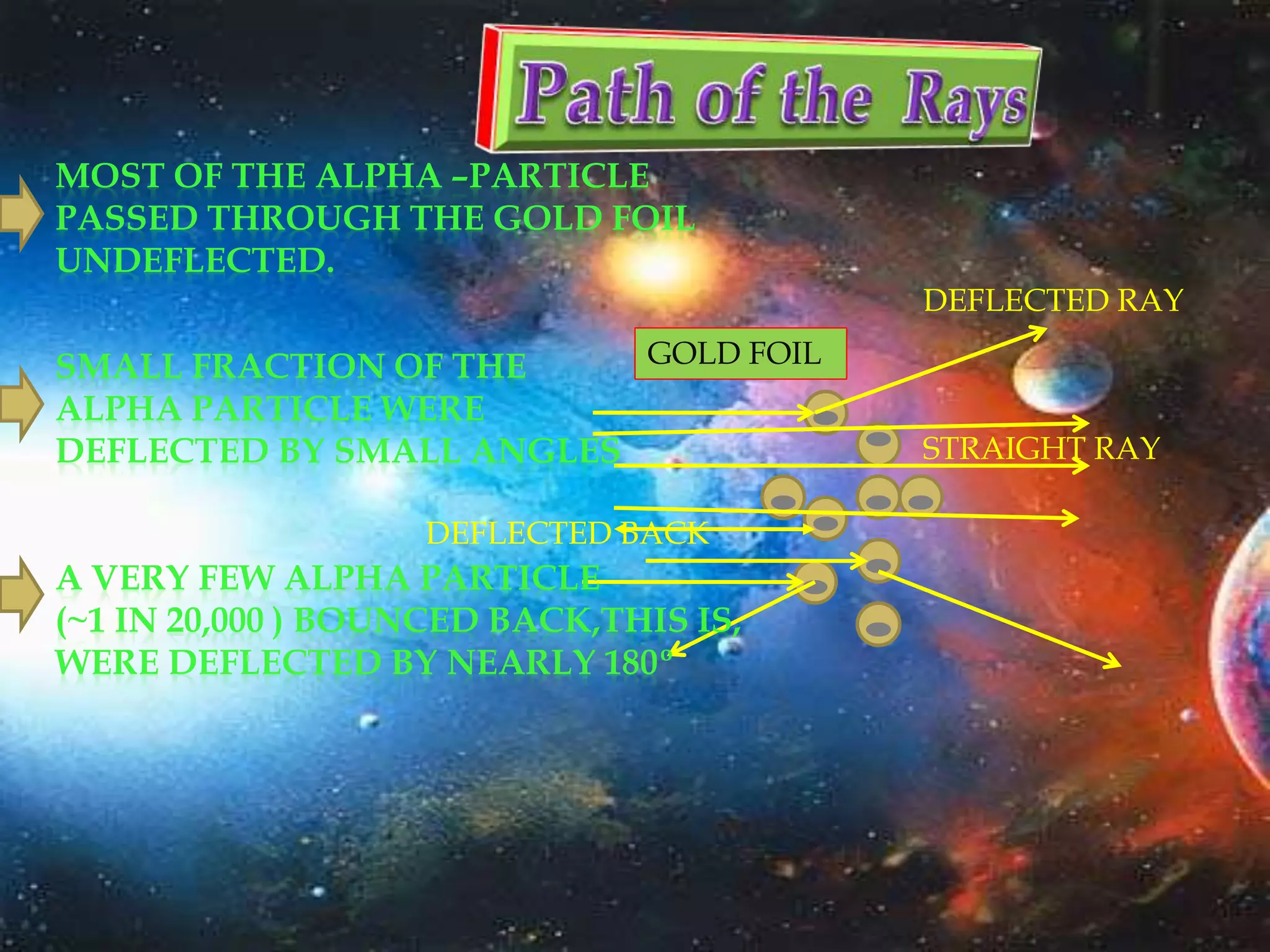
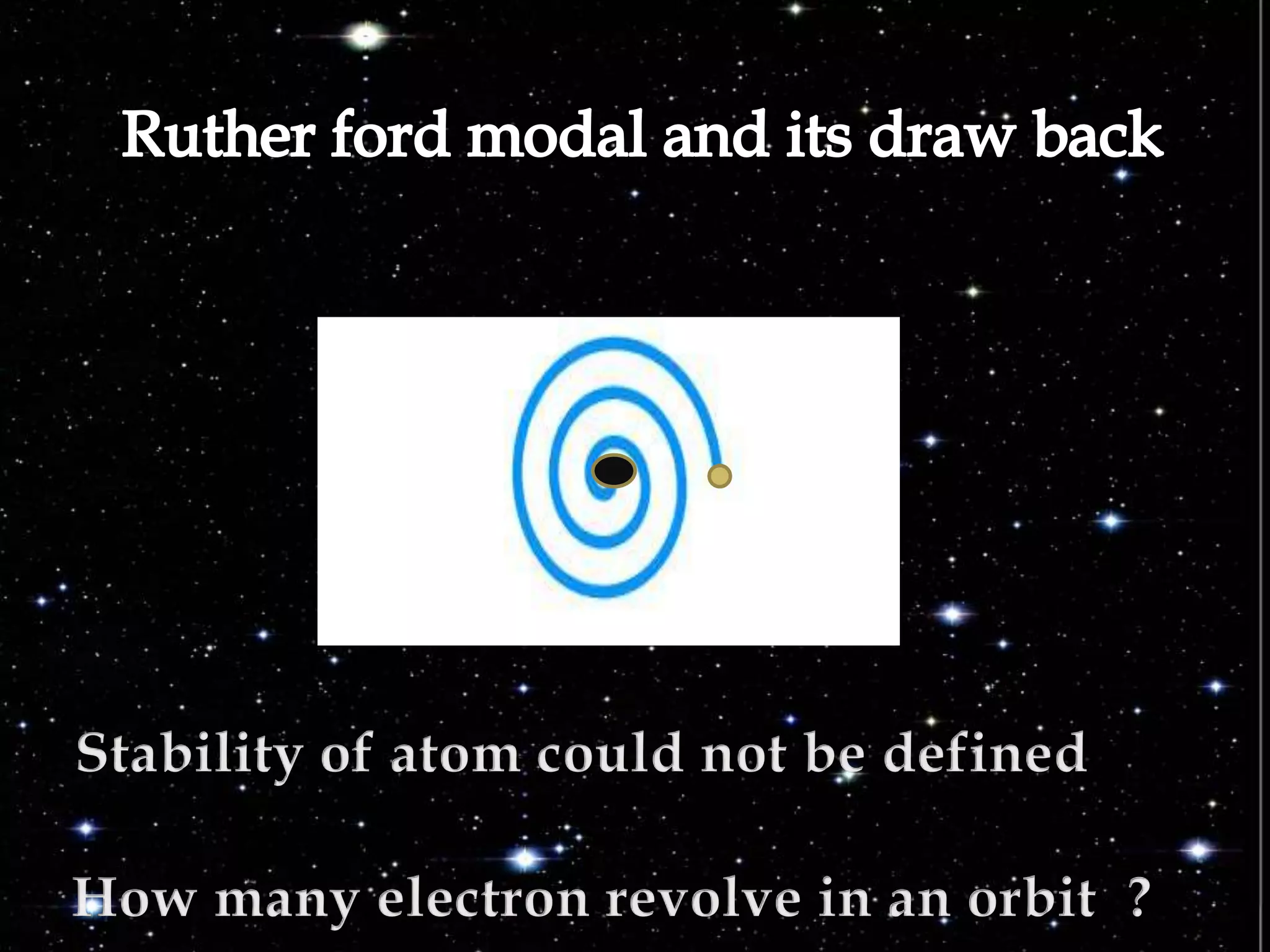
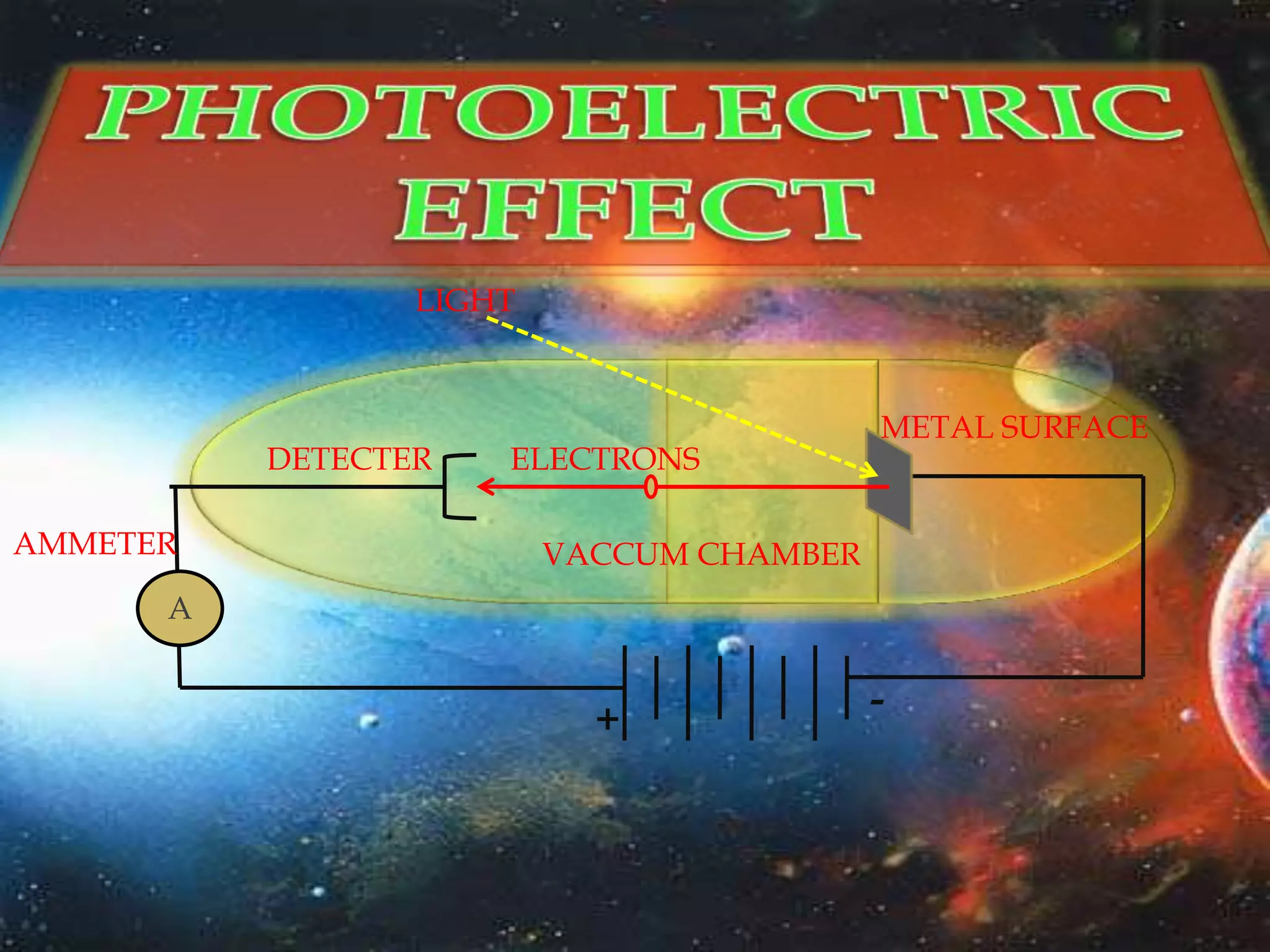
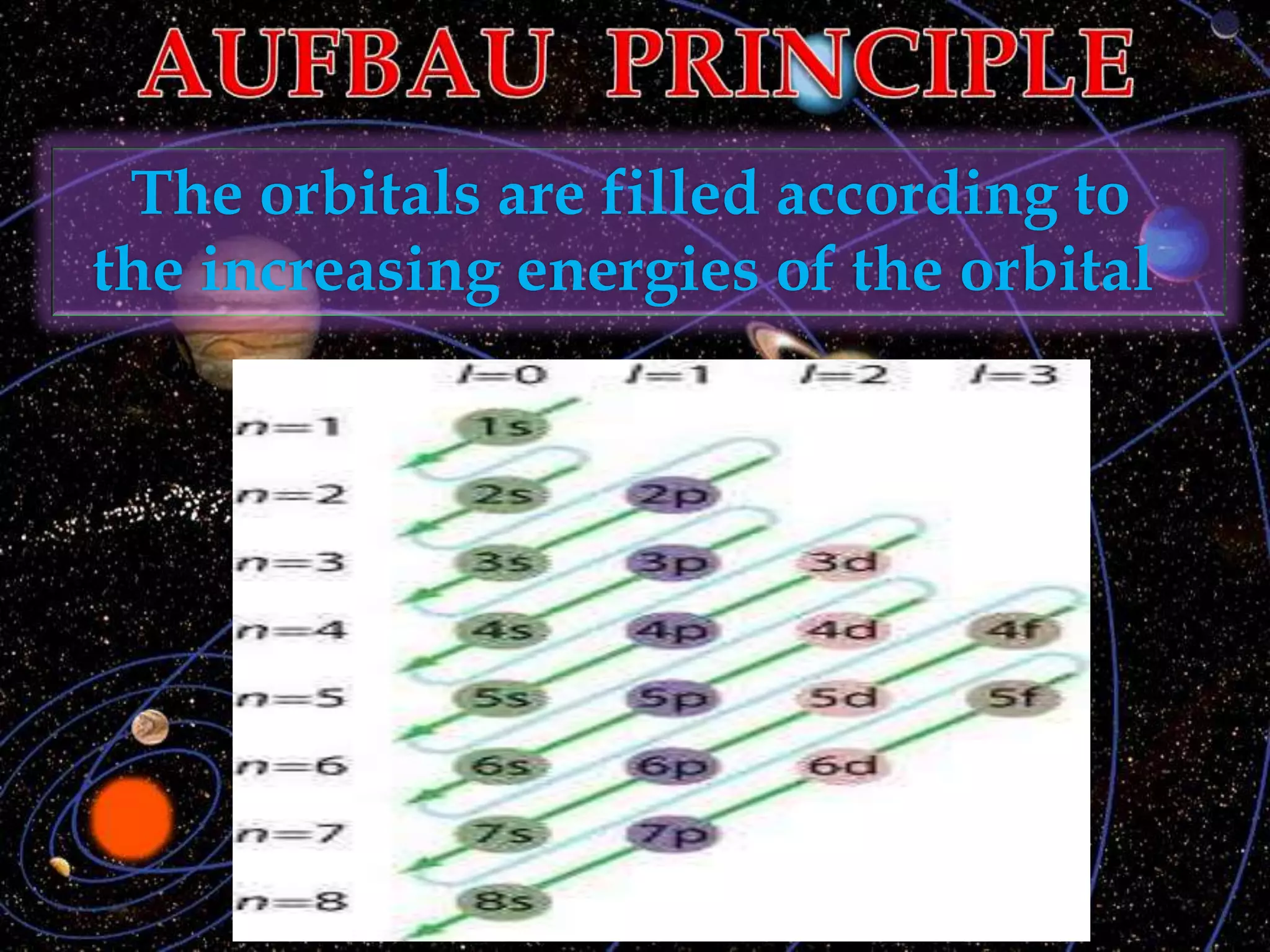
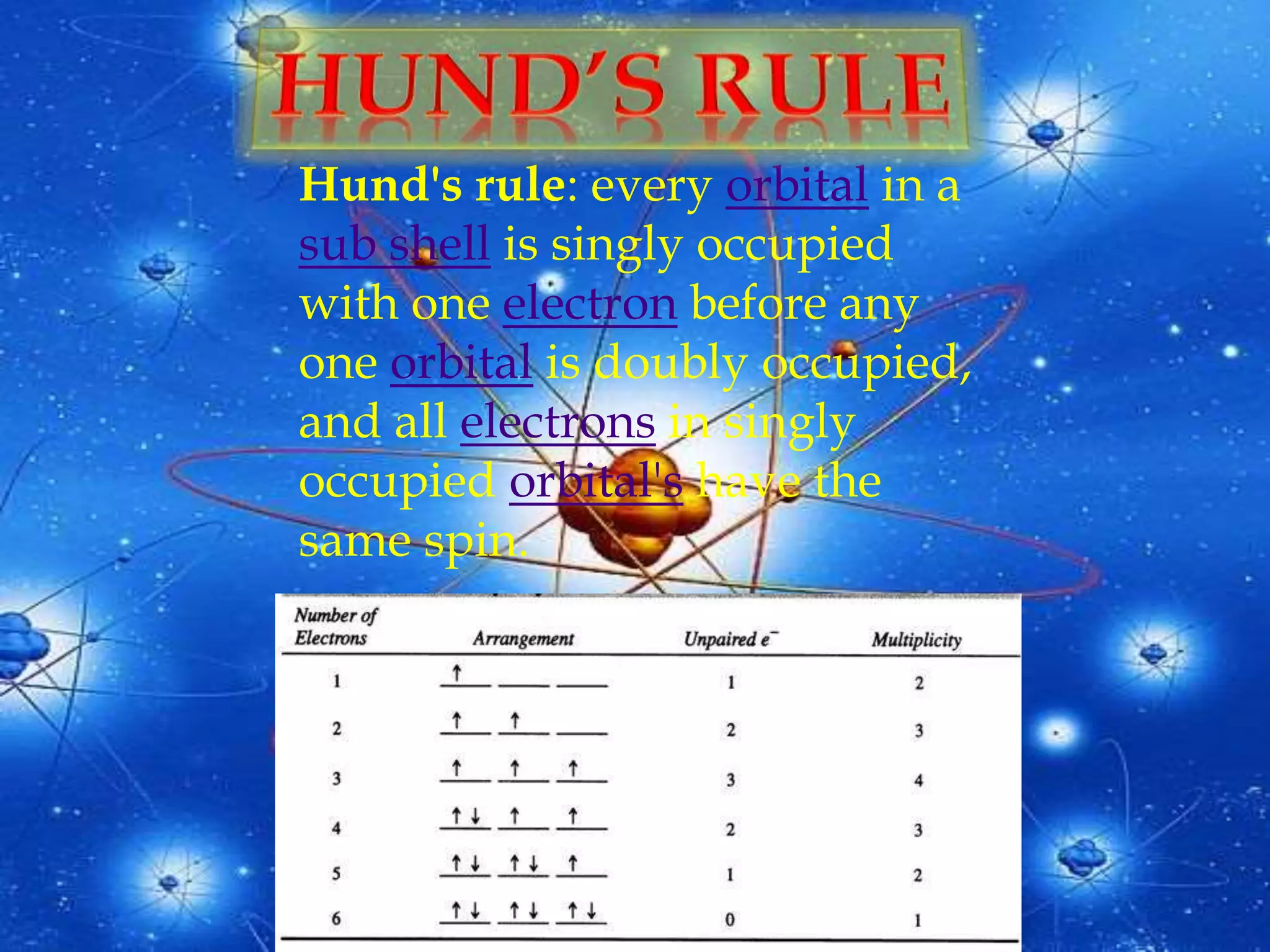
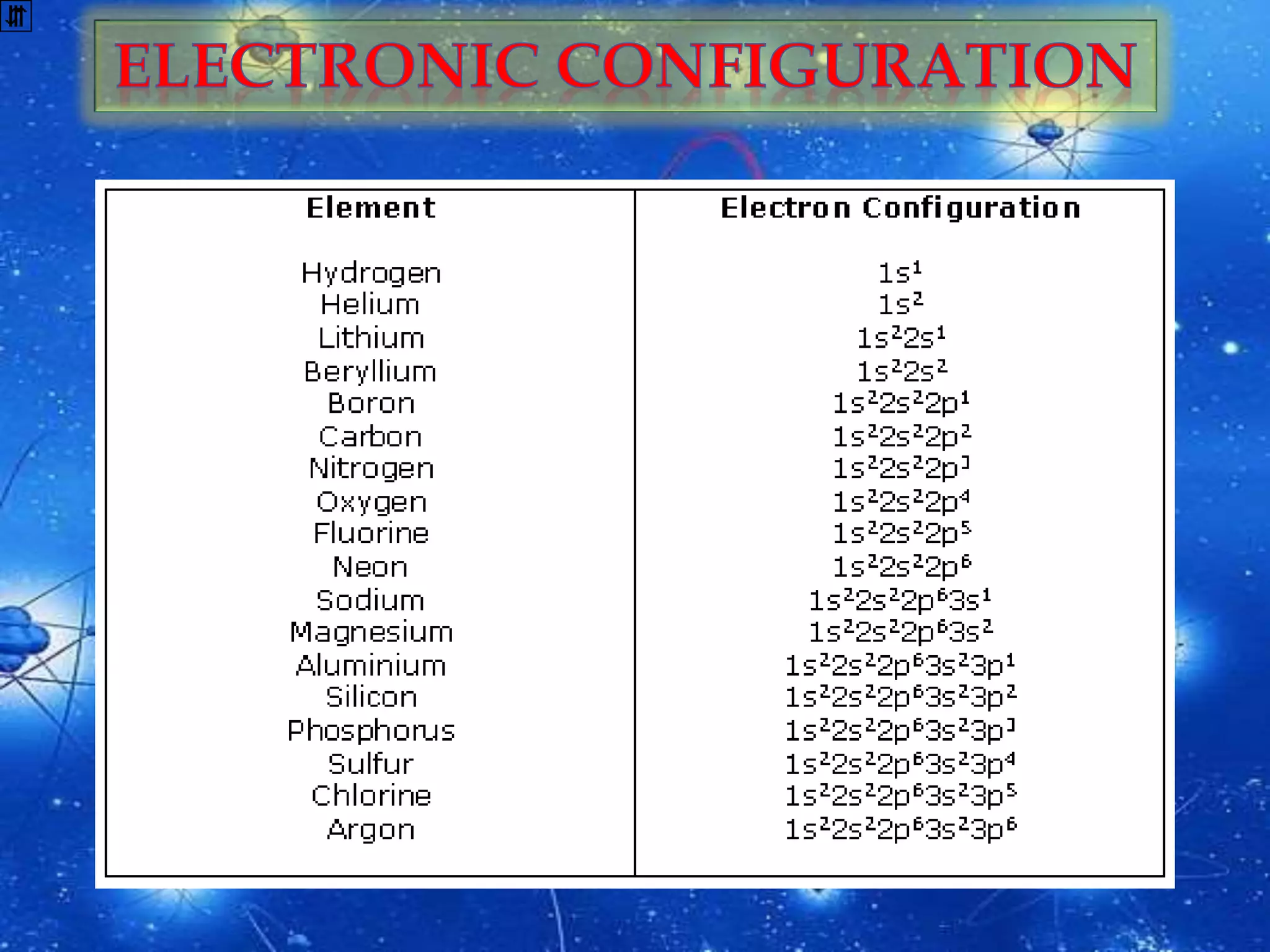
The document discusses the history of the discovery of atoms and their components. It begins with ancient Indian and Greek philosophers proposing the earliest ideas of atoms in 400 BC. In the early 1900s, scientists like J.J. Thomson discovered the electron through cathode ray experiments. Ernest Rutherford then used the gold foil experiment to show that atoms have a small, dense nucleus. This led to the modern understanding that atoms consist of a small, positively charged nucleus surrounded by electrons. Quantum physics, developed by Max Planck and others in the early 20th century, was necessary to fully explain the behavior of subatomic particles.