This document provides an overview of some foundational concepts in inorganic chemistry, including:
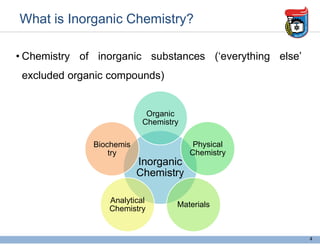
1. It defines inorganic chemistry as the study of inorganic substances excluding organic compounds.
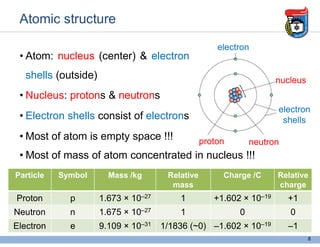
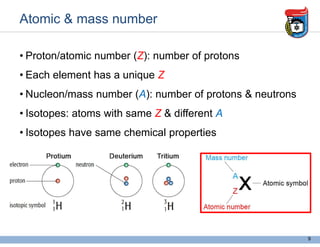
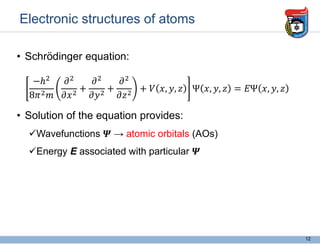
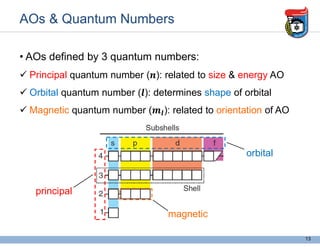
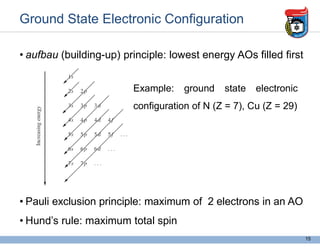
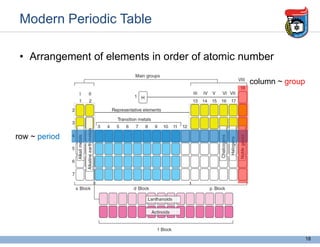
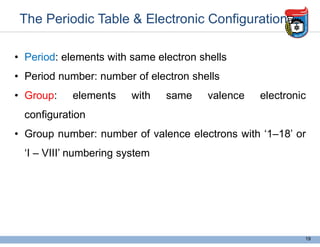
2. It discusses atomic structure, including the components of atoms like protons, neutrons, and electrons. Molecular structures and bonding are also introduced.
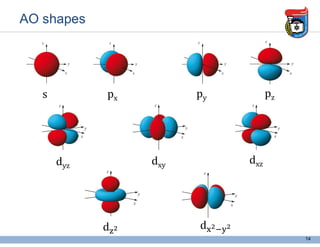
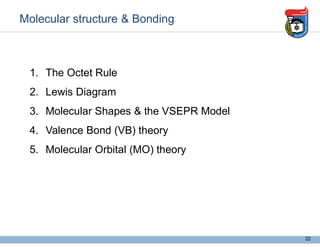
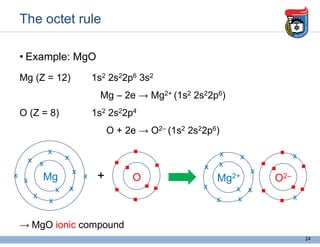
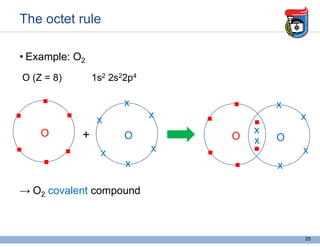
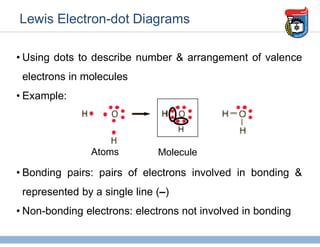
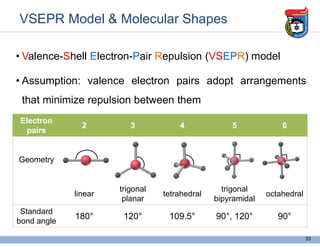
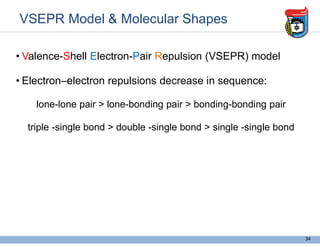
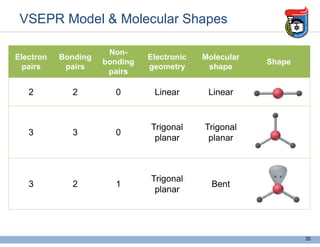
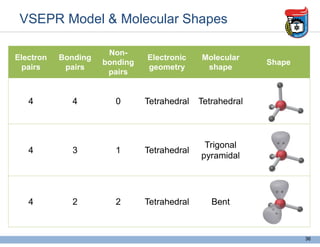
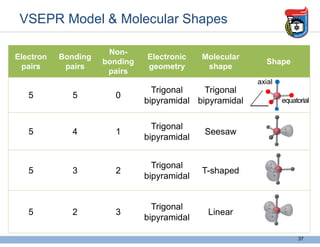
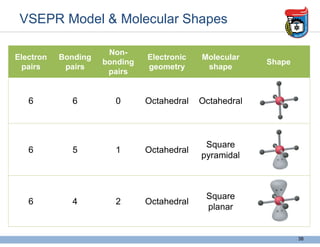
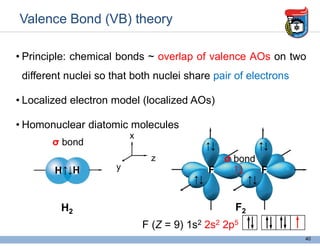
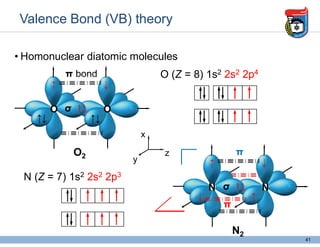
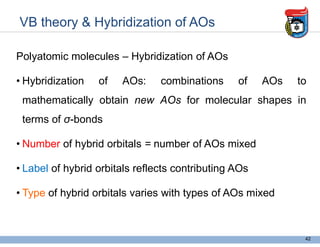
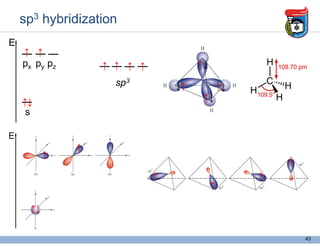
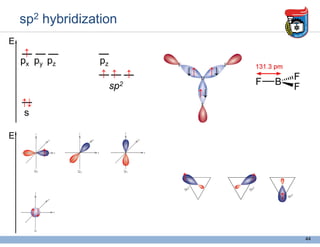
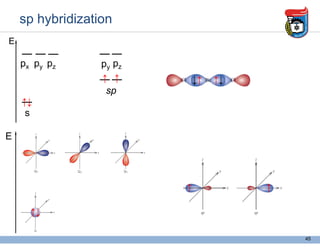
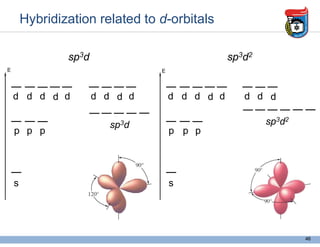
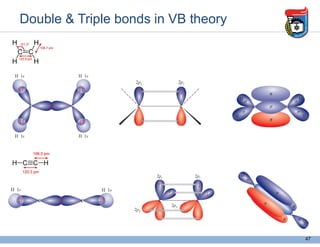
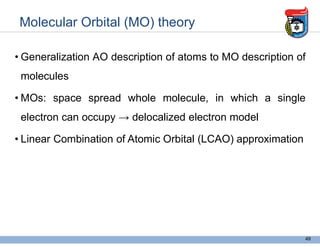
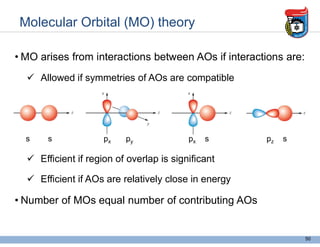
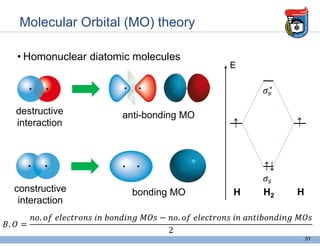
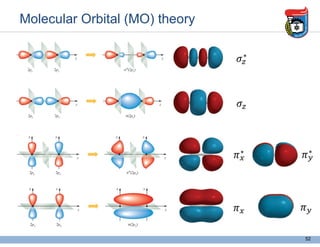
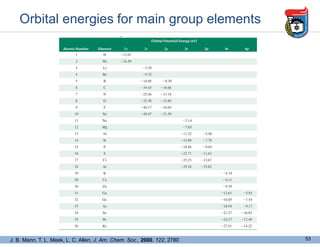
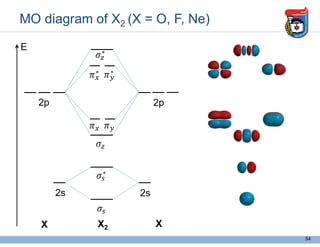
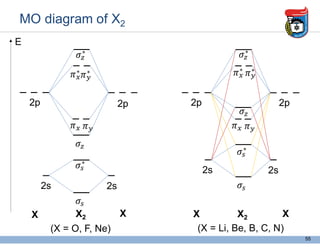
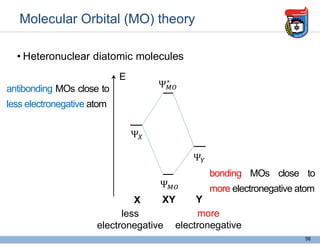
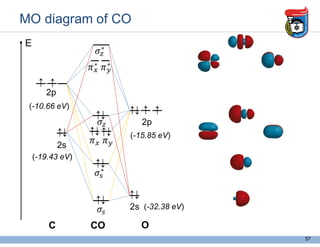
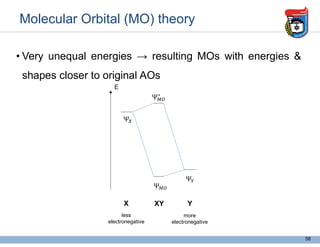
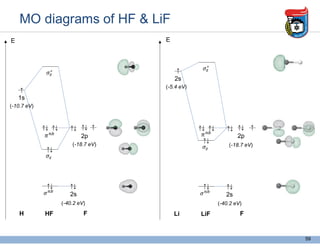
3. Key concepts in molecular structure and bonding are covered, including the octet rule, Lewis diagrams, VSEPR model for predicting molecular shapes, valence bond theory involving hybrid atomic orbitals, and molecular orbital theory involving delocalized molecular orbitals.