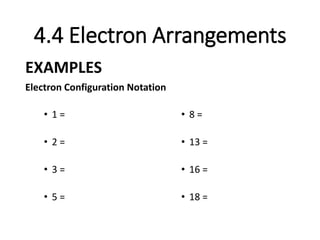
This document provides an overview of electromagnetic radiation and atomic structure concepts. It defines key terms like wavelength, frequency, electromagnetic spectrum, photon, excited and ground states. It describes how atoms emit photons of specific wavelengths when moving between quantized energy levels. Diagrams show atomic orbitals and how electrons fill different principal and subordinate energy levels based on the Aufbau principle and Hund's rule. Assignments review these topics through worksheets, labs, and practice with electron configurations.