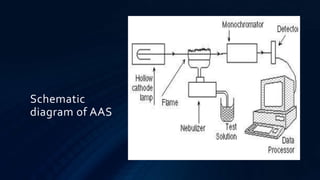
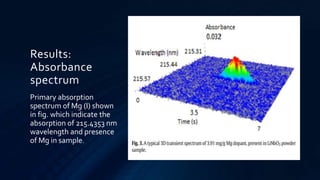
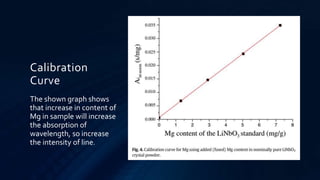
Atomic absorption spectroscopy is an analytical technique used to determine the concentration of metals in samples. It works by vaporizing the sample using a flame or graphite furnace and measuring the absorption of light from a hollow cathode lamp at specific wavelengths that correspond to the metal of interest. The amount of light absorbed is proportional to the metal concentration. The technique was developed in the 1950s and can detect over 62 elements down to low concentration levels. It has widespread applications in fields like environmental analysis, food testing, and medical diagnostics.