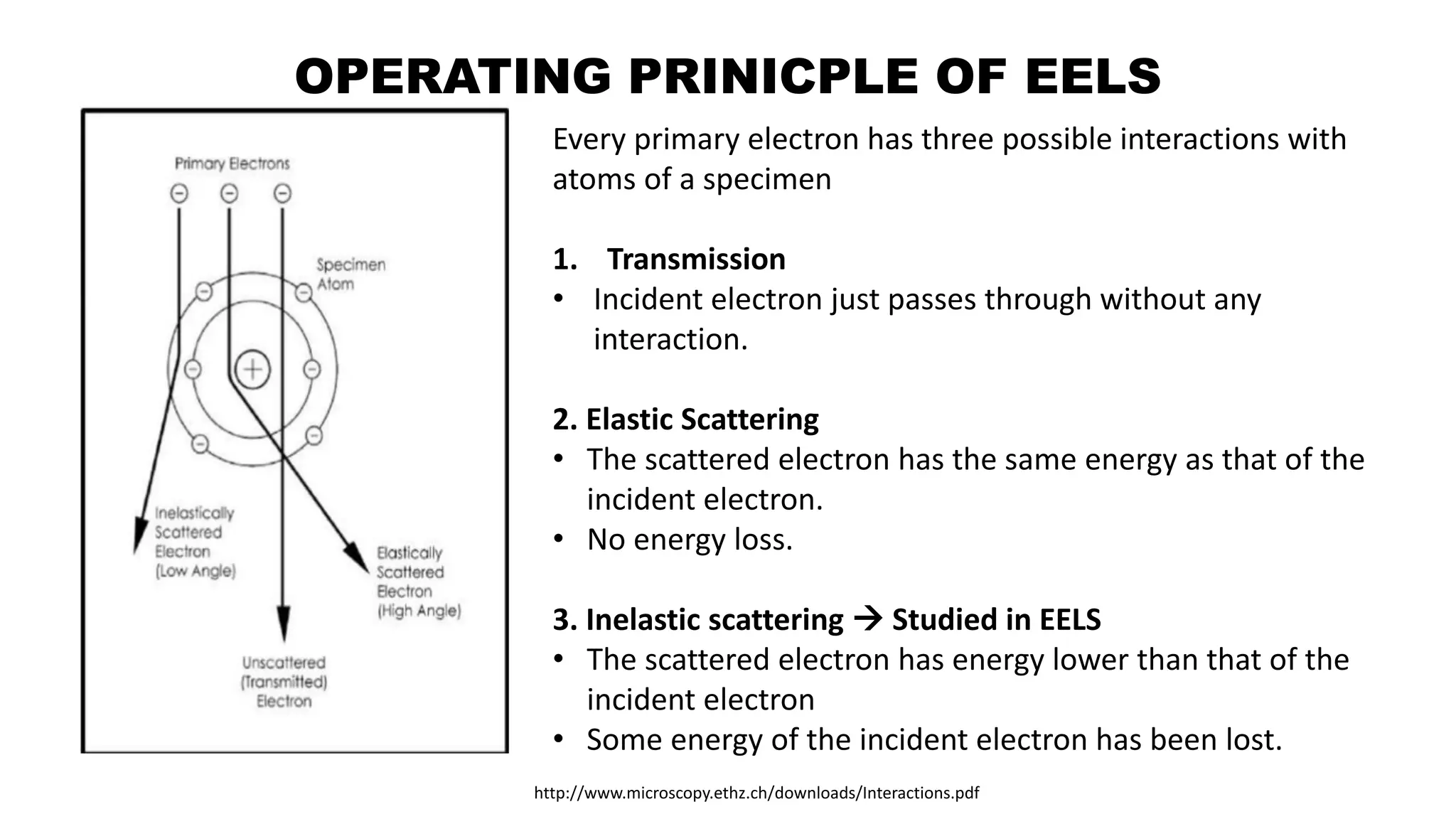
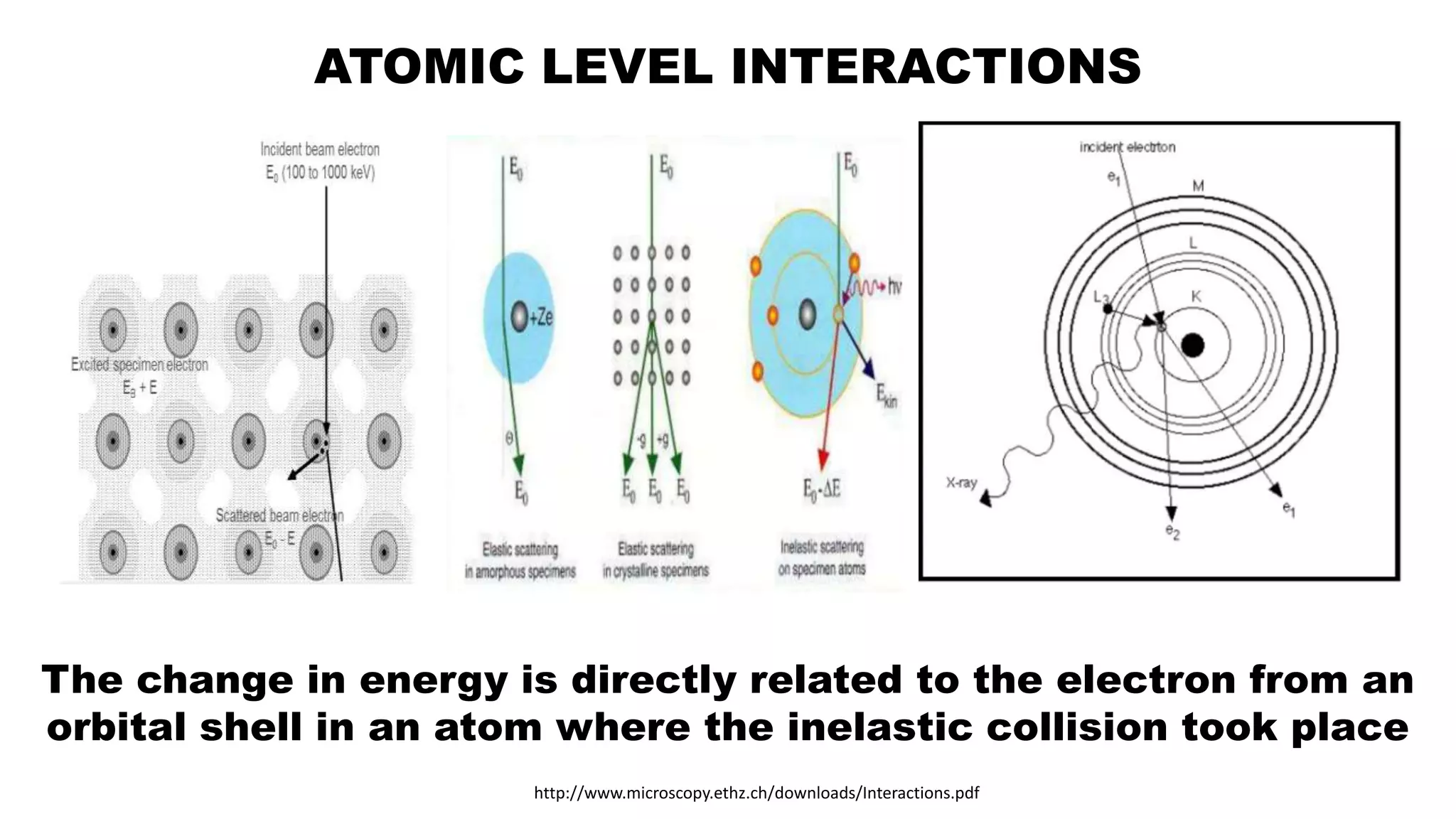
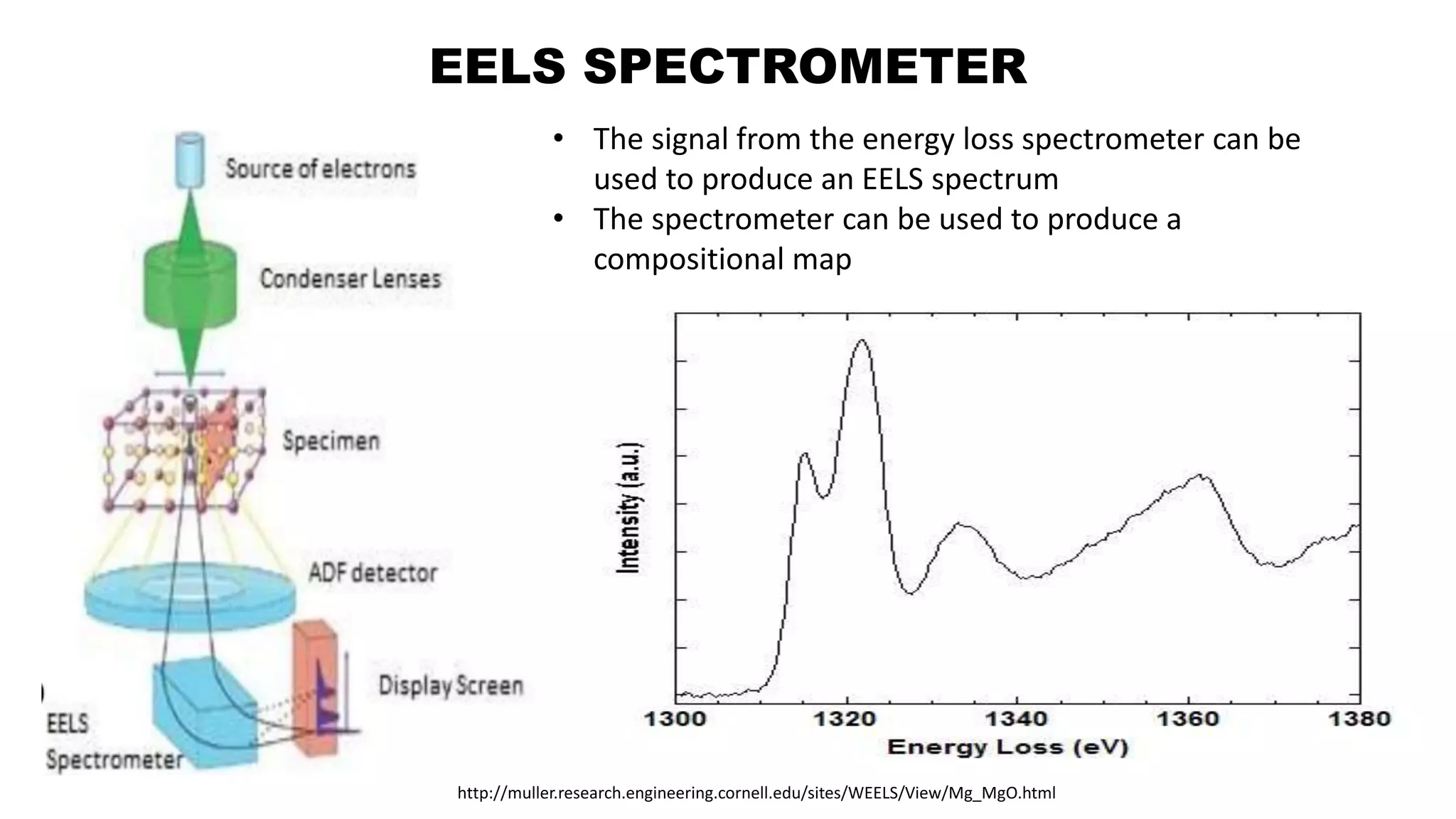
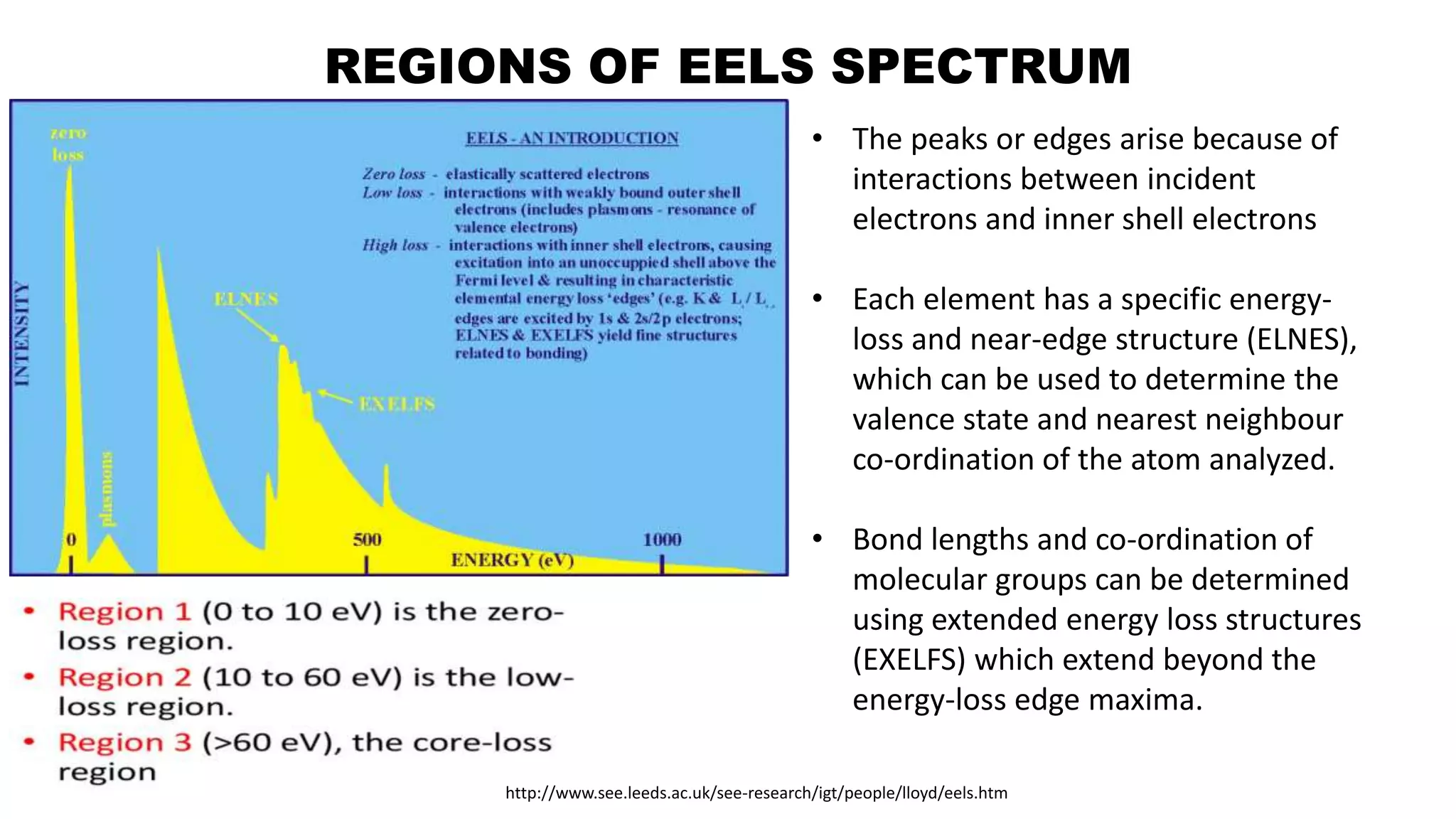
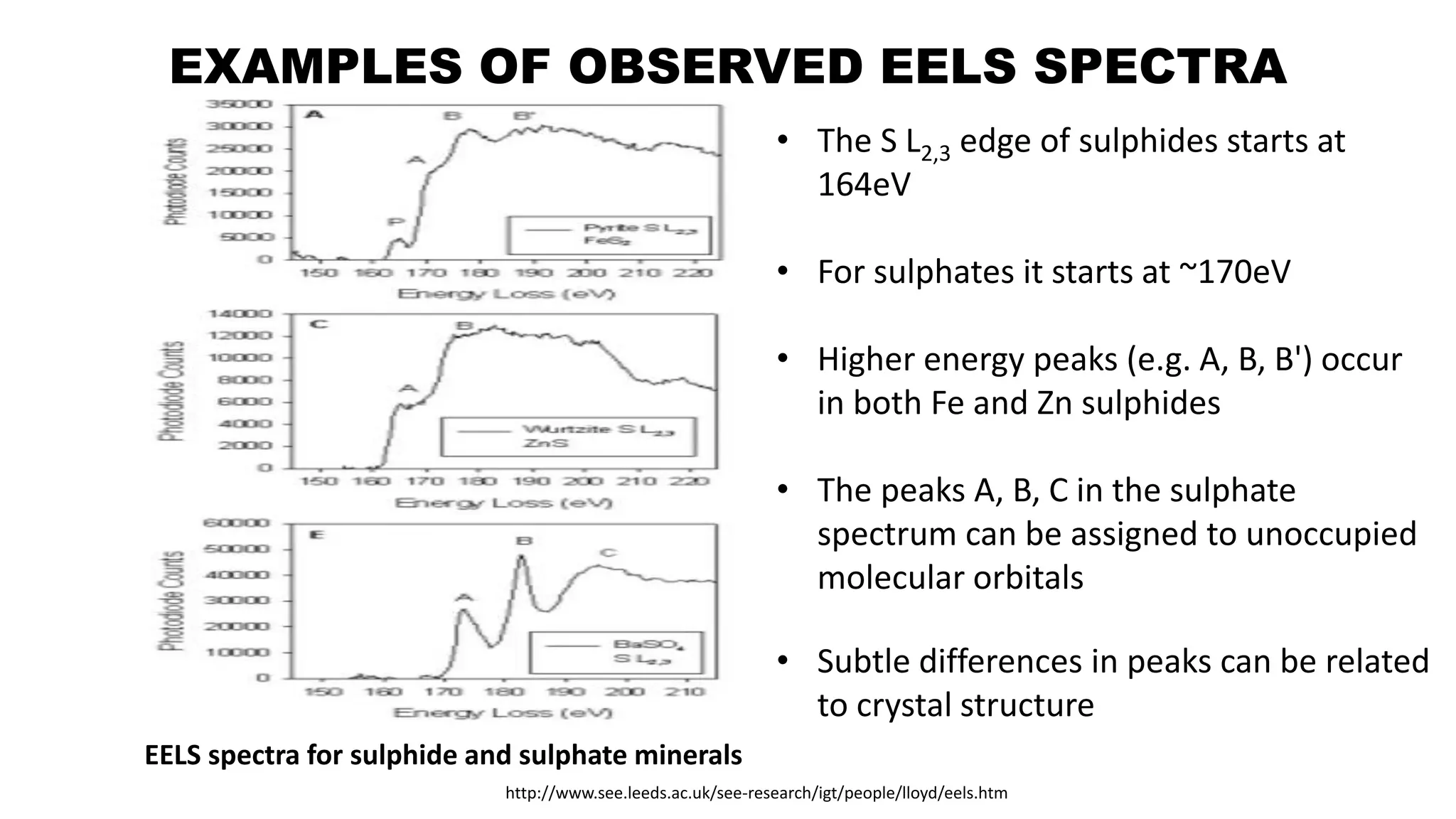
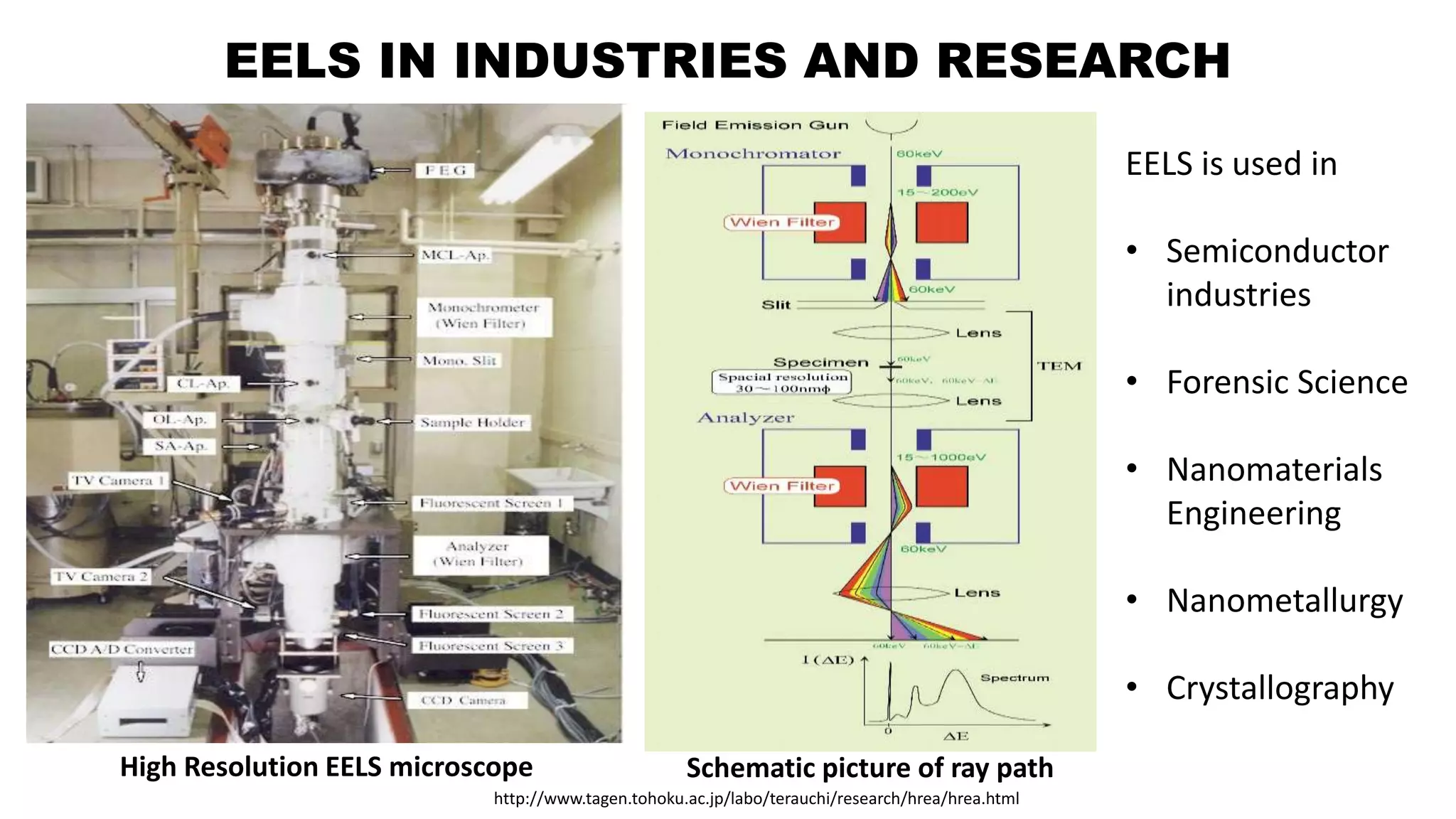
The document discusses electron energy loss spectroscopy (EELS), a technique used to study the energy loss of inelastically scattered electrons and its interaction with specimen atoms. It outlines the operating principles, regions of EELS spectrum, and applications in various fields such as semiconductor industries and forensic science. Additionally, it covers the costs associated with EELS equipment from major manufacturers.