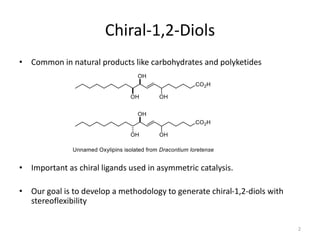
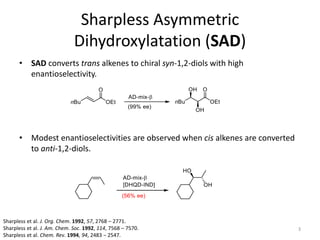
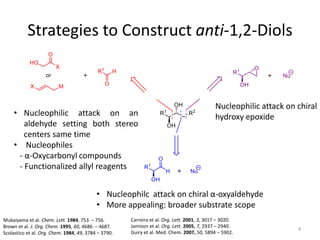
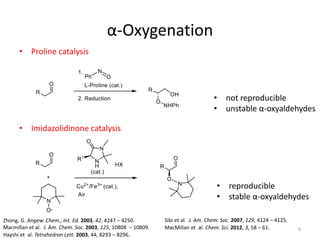
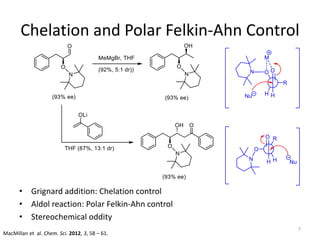
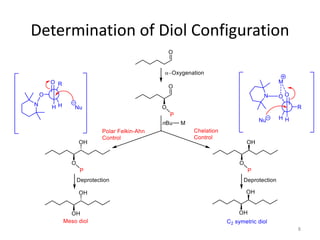
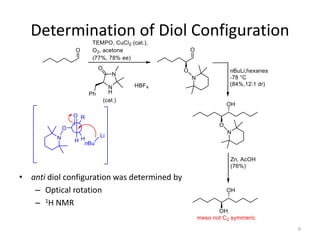
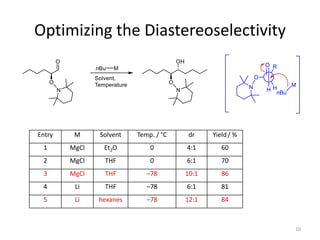
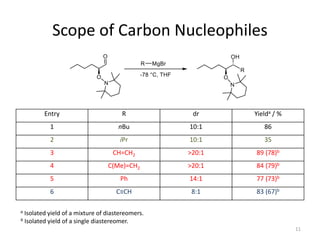
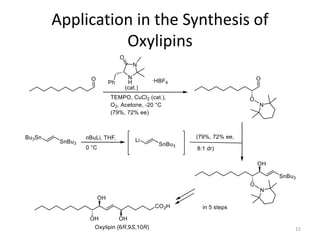
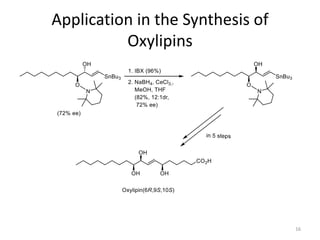
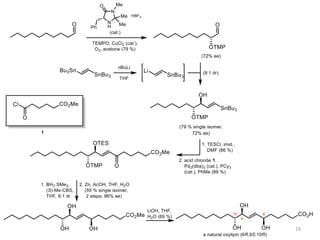
1) The document describes a methodology for synthesizing chiral anti-1,2-diols from α-oxyaldehydes using organocatalytic α-oxygenation. This provides a convenient route to access these important building blocks.
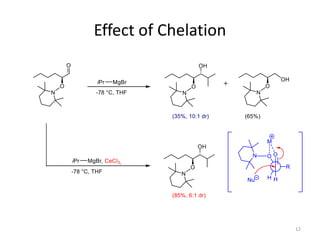
2) Key reactions include the addition of organometallic reagents like Grignard reagents to α-oxyaldehydes, which proceeds with high diastereoselectivity through chelation control.
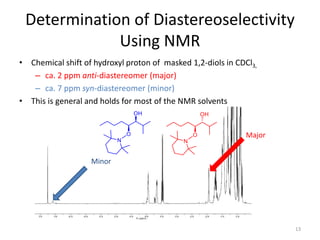
3) The anti diol configuration of products is determined by NMR spectroscopy, exploiting the difference in chemical shifts of the hydroxyl protons between the anti and syn diastereomers.