This document provides an overview of chronic kidney disease (CKD) including definitions, epidemiology, pathophysiology, risk factors, and genetics. Some key points include:
- CKD is defined as kidney damage or glomerular filtration rate <60 mL/min/1.73m2 for ≥3 months.
- It affects 14-15% of US adults and prevalence increases with age. The leading causes are hypertension and diabetes.
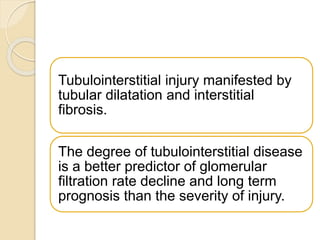
- As CKD progresses, surviving nephrons undergo hypertrophy which can lead to sclerosis and loss of filtration surface area over time. Tubulointerstitial fibrosis also contributes to declining kidney function.
- The renin-angiotensin-