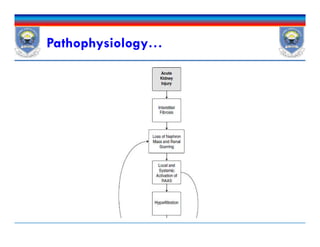
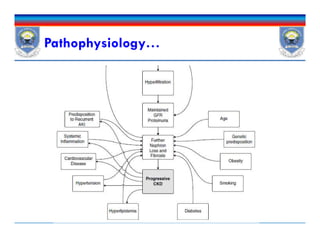
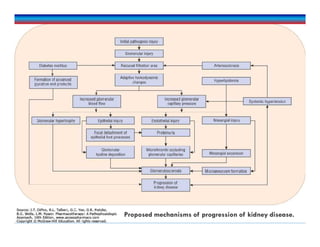
This document discusses chronic kidney disease (CKD). It defines CKD and notes that risk factors include diabetes, hypertension, glomerulonephritis, smoking, dyslipidemia, and obesity. These conditions can both initiate and promote progression of CKD by damaging kidney structures over time. The document outlines epidemiological data on CKD and provides details on how specific conditions like diabetes and hypertension increase CKD risk and progression. It also examines the role of proteinuria and other factors in contributing to declining kidney function in CKD patients.
![Renal Disorders PharmacotherapyRenal Disorders Pharmacotherapy
Chapter 3
Chronic Kidney Disease [CKD]
Renal Disorders Pharmacotherapy
By: Tsegaye Melaku
[B.Pharm, MSc, Clinical Pharmacist]
March, 2017March, 2017
tsegayemlk@yahoo.com or tsegaye.melaku@ju.edu.et
+251913765609+251913765609
Chapter 3
Chronic Kidney Disease [CKD]](https://image.slidesharecdn.com/chronickidneydisease-170323115036/75/Chronic-Kidney-Disease-CKD-1-2048.jpg)


![Epidemiology
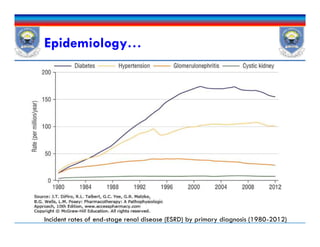
Globally, 8% to 16% of the general population has CKD
1.9 million patients are undergoing renal replacement therapy[RRT].
Prevalence of CKD increases with age: ~30% in >70 years old.
Diabetes and hypertension are also important risk factors for CKD.
In patients with type 2 diabetes, prevalence of 27%.
Among T1DM; 17% to 25% of patients developed diabetic chronic
kidney disease (DCKD) after 30 years.
Globally, 8% to 16% of the general population has CKD
1.9 million patients are undergoing renal replacement therapy[RRT].
Prevalence of CKD increases with age: ~30% in >70 years old.
Diabetes and hypertension are also important risk factors for CKD.
In patients with type 2 diabetes, prevalence of 27%.
Among T1DM; 17% to 25% of patients developed diabetic chronic
kidney disease (DCKD) after 30 years.](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-7-320.jpg)

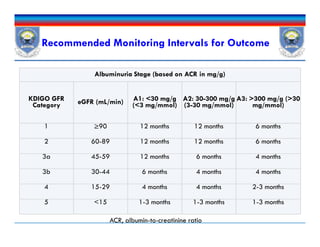
![GFR Categorya GFR (mL/min2 [mL/s/]) Terms
1 >90 (>0.87) Normal or high
2 60–89 (0.58–0.86) Mildly decreased
3a 45–59 (0.43–0.57) Mildly to moderately
decreased
CKD Stages Based on KDIGO Classification
Mildly to moderately
decreased
3b 30–44 (0.29–0.42) Moderately to severely
decreased
4 15–29 (0.14–0.28) Severely decreased
5 <15 (<0.14) Kidney failure
aTo meet criteria for CKD there must be a significant reduction in GFR (categories 3a-5) or there must
also be evidence of kidney damage (categories 1 and 2) for 3 months or greater.
CKD can be present with normal/near normal GFR if other markers of kidney disease are
present](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-28-320.jpg)

![Desired Outcome
Delay or prevent progression of the disease
Minimizing the development or severity of associated complications.
Plan for RRT(HD or PD) starting from CKD
Sustain and improve, if possible, the patient’s quality of life [CKD 5]
Prevent adverse outcomes by aggressively managing complications of
CKD.
Delay or prevent progression of the disease
Minimizing the development or severity of associated complications.
Plan for RRT(HD or PD) starting from CKD
Sustain and improve, if possible, the patient’s quality of life [CKD 5]
Prevent adverse outcomes by aggressively managing complications of
CKD.](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-34-320.jpg)
![Pharmacologic management
Proteinuria
Initiate ACEIs/ARBs
DCKD: ACEI/ARB 1st line therapy [urine albumin excretion is in
category A2 or greater (ACR between 30-300 mg/g)]
Increase dose until albuminuria is reduced by 30% to 50% or side
effects [e.g. >30% decrease in eGFR or elevation in serum potassium
occur]
ACEI + ARB ??
ACEI/ARB+ Aldosterone antagonist??
Proteinuria
Initiate ACEIs/ARBs
DCKD: ACEI/ARB 1st line therapy [urine albumin excretion is in
category A2 or greater (ACR between 30-300 mg/g)]
Increase dose until albuminuria is reduced by 30% to 50% or side
effects [e.g. >30% decrease in eGFR or elevation in serum potassium
occur]
ACEI + ARB ??
ACEI/ARB+ Aldosterone antagonist??
DCKD: Diabetic CKD](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-36-320.jpg)
![ Dihydropyridine CCBs do not appear to have any beneficial effects
beyond those attributable to reducing BP.
Nondihydropyridine agents (diltiazem and verapamil]:yielded beneficial
effects on proteinuria, although not as profoundly as ACEIs.
2nd antiproteinuric drugs [ACEI/ARB is contraindicated/not tolerated]
Suppress of glomerular hypertrophy,
Inhibit of platelet aggregation, and
Decrease in salt accumulation.
Pharmacologic management…
Dihydropyridine CCBs do not appear to have any beneficial effects
beyond those attributable to reducing BP.
Nondihydropyridine agents (diltiazem and verapamil]:yielded beneficial
effects on proteinuria, although not as profoundly as ACEIs.
2nd antiproteinuric drugs [ACEI/ARB is contraindicated/not tolerated]
Suppress of glomerular hypertrophy,
Inhibit of platelet aggregation, and
Decrease in salt accumulation.](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-37-320.jpg)

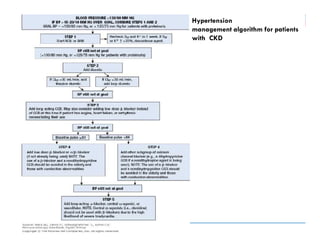
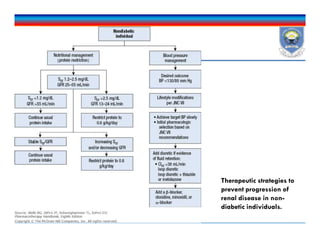
![Diabetes
Patients with DM should be screened annually for CKD
Start at the time of diagnosis of T2DM
5 years after the diagnosis of T1DM
Reduction of proteinuria and achievement of desired BP & HbA1c [7%]
CKD 3 and 4 are at higher risk of developing hypoglycemia
Reduction in metabolism of insulin by the kidney as GFR declines.
Require reduced doses of oral or injectable hypoglycemic agents
Metformin:
Continued: eGFR ≥45 mL/min; Reviewed: eGFR 30 to 44 mL/min,
Discontinued: eGFR <30 mL/min
Patients with DM should be screened annually for CKD
Start at the time of diagnosis of T2DM
5 years after the diagnosis of T1DM
Reduction of proteinuria and achievement of desired BP & HbA1c [7%]
CKD 3 and 4 are at higher risk of developing hypoglycemia
Reduction in metabolism of insulin by the kidney as GFR declines.
Require reduced doses of oral or injectable hypoglycemic agents
Metformin:
Continued: eGFR ≥45 mL/min; Reviewed: eGFR 30 to 44 mL/min,
Discontinued: eGFR <30 mL/min](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-42-320.jpg)
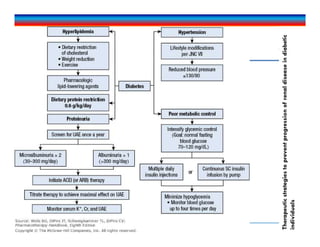
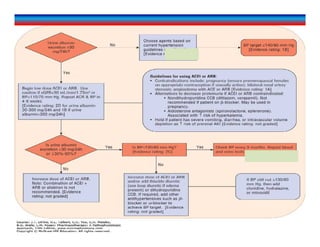
![Recommendations
Nonpharmacologic
Exercise 30 minutes five times per week [1D]
Weight loss if BMI >25 kg/m2 [1D]
Smoking cessation [1D]
Alcohol: Two standard drinks per day for men and one standard drink per day for women[2D]
If hypertension: Low-sodium diet (<2 g/day, <90 mmol/day) [1C]
Pharmacologic
Adjust medication doses for kidney function [1A]
Seek pharmacist or medical advice before using over-the-counter medicines or nutritional protein
supplements [1B]
Herbal medicines are not recommended [1B]
Temporarily discontinue potentially nephrotoxic/renally excreted drugs if eGFR <60 mL/min/1.73
m2 in patients who are acutely unwell or hypovolemic (eg, metformin, RAAS blockers, diuretics,
NSAIDs/COX II inhibitors, lithium, digoxin) [1C]
Vaccines:
Influenza yearly [1B]
Pneumococcal vaccine if eGFR <30 mL/min/1.73 m2, nephrotic syndrome, diabetes, or receiving
immunosuppression. Single booster dose at year 5 [1B]
Hepatitis B vaccine if eGFR <30 mL/min/1.73 m2 and risk of progression of CKD [1B]
ASA suggested for patients at risk for atherosclerotic events unless there is an increased bleeding
risk [2B]
Avoid oral phosphate-containing bowel preparations in people with a GFR <60 mL/min/1.73
m2 (<0.58 mL/s/m2) or in those known to be at risk of phosphate nephropathy [1A]
Pharmacologic
Adjust medication doses for kidney function [1A]
Seek pharmacist or medical advice before using over-the-counter medicines or nutritional protein
supplements [1B]
Herbal medicines are not recommended [1B]
Temporarily discontinue potentially nephrotoxic/renally excreted drugs if eGFR <60 mL/min/1.73
m2 in patients who are acutely unwell or hypovolemic (eg, metformin, RAAS blockers, diuretics,
NSAIDs/COX II inhibitors, lithium, digoxin) [1C]
Vaccines:
Influenza yearly [1B]
Pneumococcal vaccine if eGFR <30 mL/min/1.73 m2, nephrotic syndrome, diabetes, or receiving
immunosuppression. Single booster dose at year 5 [1B]
Hepatitis B vaccine if eGFR <30 mL/min/1.73 m2 and risk of progression of CKD [1B]
ASA suggested for patients at risk for atherosclerotic events unless there is an increased bleeding
risk [2B]
Avoid oral phosphate-containing bowel preparations in people with a GFR <60 mL/min/1.73
m2 (<0.58 mL/s/m2) or in those known to be at risk of phosphate nephropathy [1A]
1=recommended; 2= suggested;; A=high quality, B=Moderate, C= Low; D= Very low](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-44-320.jpg)
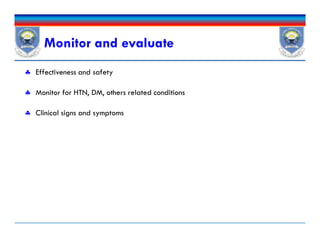
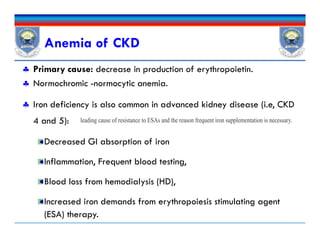

![ Sign & sxs of anemia:
Fatigue, shortness of breath, cold intolerance, chest pain, tingling in
the extremities, tachycardia, headaches, and general malaise.
CBC: Hb concentrations [<13 g/dL for male & <12 g/dL for females]
Annually in CKD 3,
biannually in CKD 4-5, and
At least every 3 months in CKD 5 patients.
Iron status; Tsat, serum ferritin
Vitamin B12 or folate, Guaiac test
Anemia of CKD…Dx
Sign & sxs of anemia:
Fatigue, shortness of breath, cold intolerance, chest pain, tingling in
the extremities, tachycardia, headaches, and general malaise.
CBC: Hb concentrations [<13 g/dL for male & <12 g/dL for females]
Annually in CKD 3,
biannually in CKD 4-5, and
At least every 3 months in CKD 5 patients.
Iron status; Tsat, serum ferritin
Vitamin B12 or folate, Guaiac test](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-52-320.jpg)
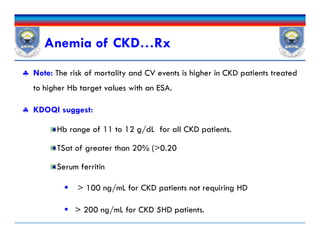
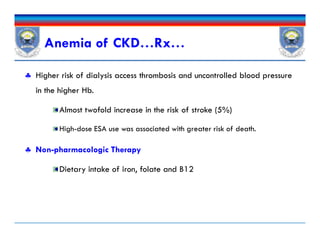
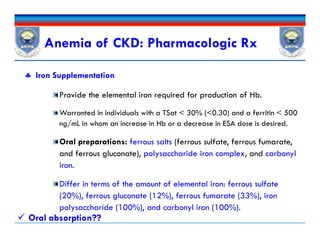


![Iron Drug Interactions
Iron absorption is decreased by
Other elements (eg, calcium in calcium-containing phosphate
binders),
Medications that increase the pH of the GI tract such as PPIs and
H2As , and antibiotics [doxycycline and tetracycline].
Iron also decreases absorption of other drugs such as antibiotics
(fluoroquinolones, doxycycline)
Iron absorption is decreased by
Other elements (eg, calcium in calcium-containing phosphate
binders),
Medications that increase the pH of the GI tract such as PPIs and
H2As , and antibiotics [doxycycline and tetracycline].
Iron also decreases absorption of other drugs such as antibiotics
(fluoroquinolones, doxycycline)](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-60-320.jpg)


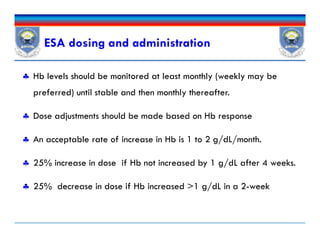
![ESA Adverse Effects
Hypertension, Hypertensive encephalopathy.
Don’t be used in those with uncontrolled blood pressure.
Seizures: within 3months of initiation
Thrombosis of the HD vascular access site and other thromboembolic
events esp. in higher hemoglobin [>13 g/dL]
ESAs are not indicated in patients receiving myelosuppressive
chemotherapy when the anticipated outcome is cure.
Hypertension, Hypertensive encephalopathy.
Don’t be used in those with uncontrolled blood pressure.
Seizures: within 3months of initiation
Thrombosis of the HD vascular access site and other thromboembolic
events esp. in higher hemoglobin [>13 g/dL]
ESAs are not indicated in patients receiving myelosuppressive
chemotherapy when the anticipated outcome is cure.](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-63-320.jpg)
![KDIGO ESA use recommendation
ND-CKD CKD 5HD and CKD 5PD Pediatric CKD
ESA initiation If Hb <10 g/dL (<100 g/L; <6.21
mmol/L). Consider rate of fall of Hb,
prior response to iron, risk of needing a
transfusion, risk of ESA therapy, and
presence of anemia symptoms before
initiating an ESA. [2C]
Do not initiate if Hb ≥10 g/dL (≥100
g/L; ≥6.21 mmol/L). [2D]
Use ESAs to avoid drop in Hb to <9
g/dL (<90 g/L; <5.59 mmol/L) by
starting an ESA when Hb is between
9 and 10 g/dL (90 and 100 g/L; 5.59
and 6.21 mmol/L). [2B]
Selection of Hb concentration at which to
initiate ESA therapy should include
consideration of potential benefits (eg,
improvement in QOL, school attendance,
avoidance of blood transfusions) and
potential harms. [2D]
If Hb <10 g/dL (<100 g/L; <6.21
mmol/L). Consider rate of fall of Hb,
prior response to iron, risk of needing a
transfusion, risk of ESA therapy, and
presence of anemia symptoms before
initiating an ESA. [2C]
Do not initiate if Hb ≥10 g/dL (≥100
g/L; ≥6.21 mmol/L). [2D]
Hb level Do not use ESAs
to intentionally increase Hb above 13
g/dL (130 g/L, 8.07 mmol/L). [1A]
Do not use ESAs to maintain Hb above
11.5 g/dL (115 g/L; 7.14 mmol/L). [2C]
Do not use ESAs
to intentionally increase Hb above 13
g/dL (130 g/L, 8.07 mmol/L). [1A]
Do not use ESAs to maintain Hb
above 11.5 g/dL (115 g/L; 7.14
mmol/L). [2C]
Suggest Hb range of 11-12 g/dL (110-120
g/L, 6.83-7.45 mmol/L). [2D]
Iron initiationb If TSat is ≤30% (≤0.30) and ferritin is
≤500 ng/mL (μg/L; ≤1,120 pmol/L).
[2C]
If TSat is ≤30% (≤0.30) and ferritin is
≤500 ng/mL (μg/L; ≤1,120 pmol/L).
[2C]
If TSat is ≤20% (≤0.20) and ferritin is
≤100 ng/mL (μg/L; ≤225 pmol/L). [1D]
CKD, chronic kidney disease; ESA, erythropoiesis
stimulating agent; Hb, hemoglobin; ND-CKD, non-
dialysis CKD patients; QOL, quality of life; TSat,
transferrin saturation.
Serum ferritin is an acute-phase reactant-use
clinical judgment when above 500 ng/mL](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-66-320.jpg)
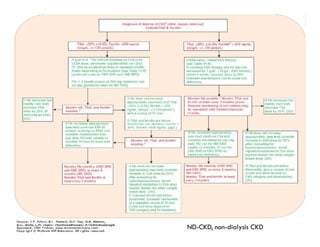


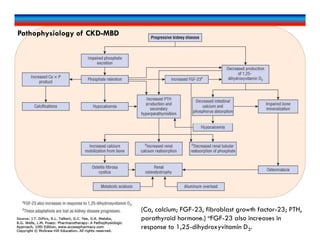
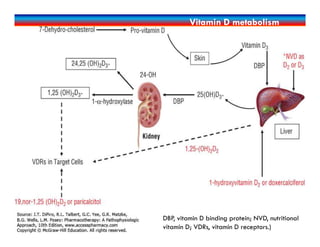
![Dx of CKD-MBD
Alterations in serum phosphorus, calcium, PTH, and 25(OH)D.
Evaluation of bone architecture
Gold standard: bone biopsy for histologic analysis[very invasive]
Other: based on patient presentation
Alterations in serum phosphorus, calcium, PTH, and 25(OH)D.
Evaluation of bone architecture
Gold standard: bone biopsy for histologic analysis[very invasive]
Other: based on patient presentation](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-74-320.jpg)
![CKD-MBD… Non-pharmacologic RX
Dietary phosphorus restriction:
To manage hyperphosphatemia
Should be initiated for most patients with CKD 3-5.
Phosphorus restriction to 800 to 1,000 mg/day
Parathyroidectomy: therapeutic option
Persistently elevated PTH associated with hypercalcemia and/or
hyperphosphatemia [refractory to medical therapy]
Dietary phosphorus restriction:
To manage hyperphosphatemia
Should be initiated for most patients with CKD 3-5.
Phosphorus restriction to 800 to 1,000 mg/day
Parathyroidectomy: therapeutic option
Persistently elevated PTH associated with hypercalcemia and/or
hyperphosphatemia [refractory to medical therapy]
When PTH >800 pg/mL](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-76-320.jpg)
![ Phosphate-Binding Agents
Especially for those with ESRD; to limit GI absorption and
thereby control serum phosphorus.
Includes: elemental calcium, iron, and lanthanum-containing
compounds, and the non-elemental agent [sevelamer]
Vitamin D Therapy
Calcimimetics: Cinacalcet acts by increasing the sensitivity of the
calcium-sensing receptor.
CKD-MBD… Pharmacologic RX
Phosphate-Binding Agents
Especially for those with ESRD; to limit GI absorption and
thereby control serum phosphorus.
Includes: elemental calcium, iron, and lanthanum-containing
compounds, and the non-elemental agent [sevelamer]
Vitamin D Therapy
Calcimimetics: Cinacalcet acts by increasing the sensitivity of the
calcium-sensing receptor.](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-77-320.jpg)
![Management of Dyslipidemia in CKD
Dyslipidemia Goal Initial Therapy Modification in
Therapya Alternativea
TG ≥500
mg/dL
TG<500
mg/dL
TLC TLC + fibrate
or niacin
Fibrate or niacin
LDL 100–129
mg/dL
LDL <100
mg/dL
TLC TLC + low-
dose statin
Bile acid
sequestrant
or niacin
LDL 100–129
mg/dL
LDL <100
mg/dL
TLC + low-
dose statin
Bile acid
sequestrant
or niacin
LDL ≥130
mg/dL
LDL <100
mg/dL
TLC + low-
dose statin
TLC +
maximum-
dose statin
Bile acid
sequestrant
or niacin
TG ≥200
mg/dL and non-
HDL
≥130mg/dL
Non-HDL <130
mg/dL
TLC + low-
dose statin
TLC +
maximum-
dose statin
Fibrate or niacin
aDosing of selected agents by class: fibrate (gemfibrozil 600 mg twice daily); niacin (1.5–3 g/day of immediate-release
product); statin (simvastatin 10–40 mg/day if GFR<30 mL/min [<0.50 mL/s], 20–80 mg/day if GFR >30 mL/min [>0.50
mL/s]); bile acid sequestrant (cholestyramine 4–16 g/day).](https://image.slidesharecdn.com/chronickidneydisease-170323115036/85/Chronic-Kidney-Disease-CKD-79-320.jpg)
