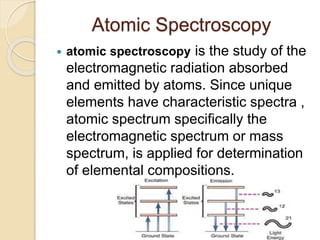
Spectroscopy is a branch of science that deals with the interaction of matter and electromagnetic radiation. Some key types of spectroscopy described in the document are atomic spectroscopy, which analyzes the radiation absorbed and emitted by atoms to determine elemental composition. Infrared spectroscopy analyzes absorption in the infrared region to provide information about chemical structure. Raman spectroscopy is a non-destructive technique that provides detailed chemical information based on the interaction of light with chemical bonds. Spectroscopy has various applications including cure monitoring of composites, estimating weathered wood exposure times, measuring toxic compounds in blood, and non-destructive elemental analysis.