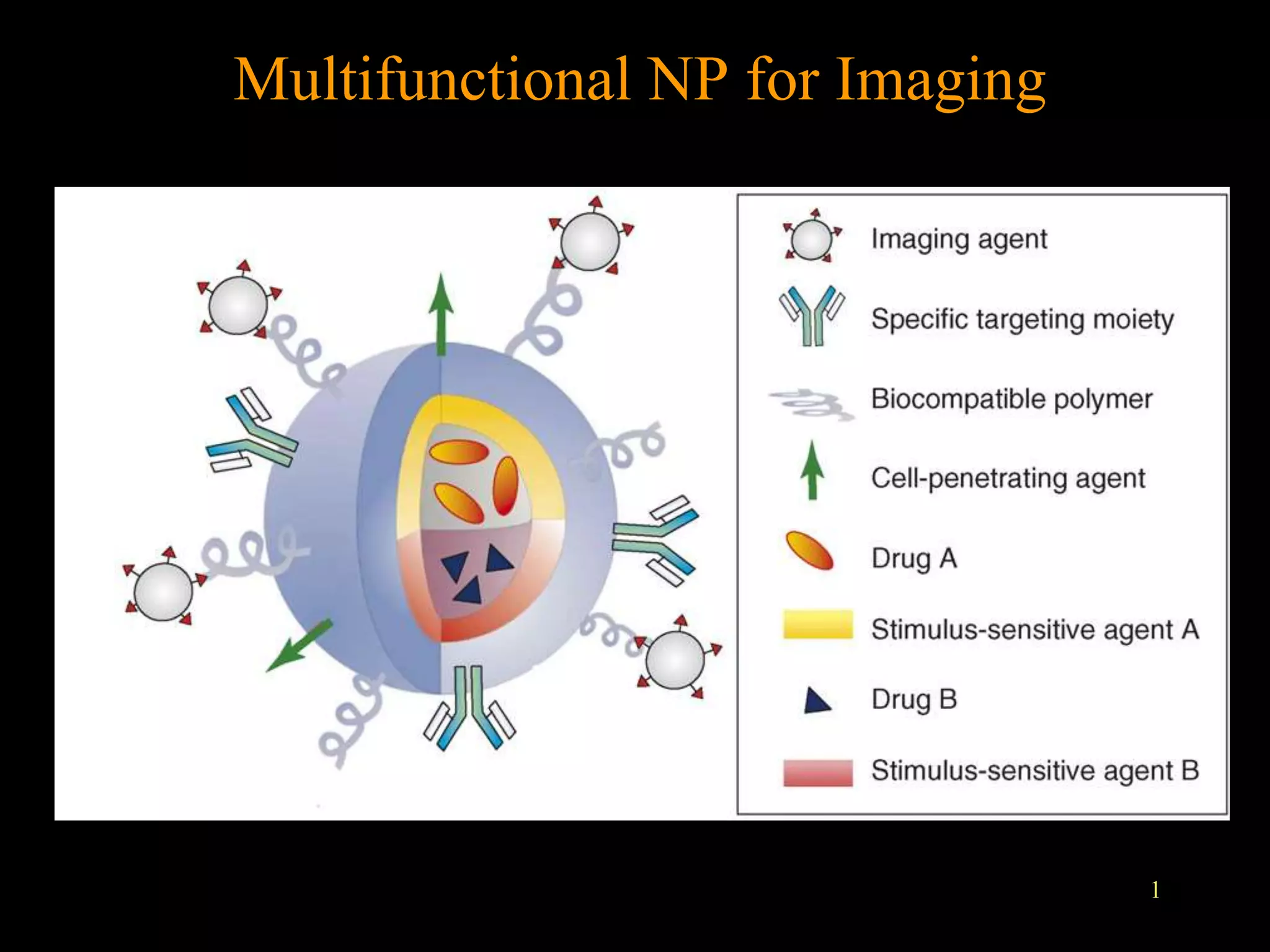
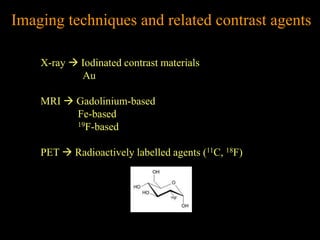
1. The document discusses various nanoparticles (NPs) and their applications in medical imaging techniques such as X-ray CT, PET, and MRI. Gold NPs and iron oxide NPs are highlighted.
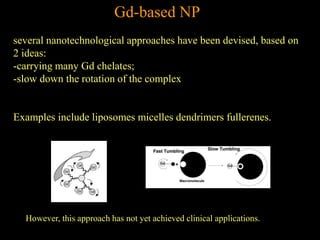
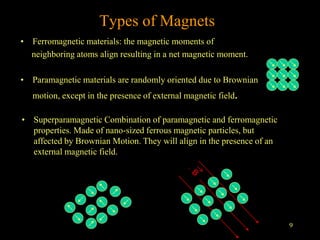
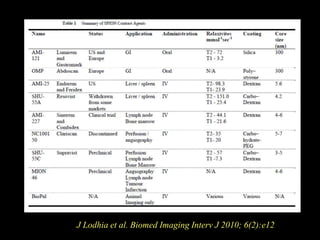
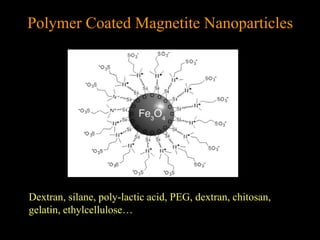
2. For MRI, iron oxide NPs can act as contrast agents by enhancing the relaxation of water protons. Superparamagnetic iron oxide NPs consisting of a magnetite or maghemite core coated with dextran or polymers are promising MRI contrast agents.
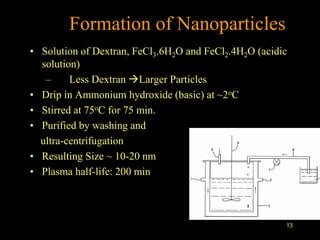
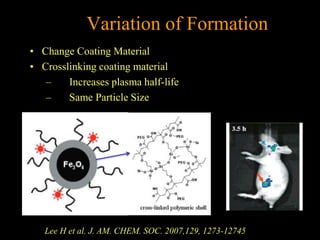
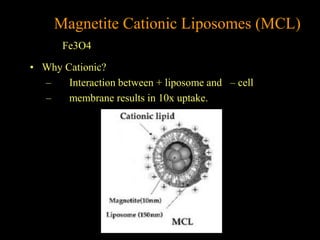
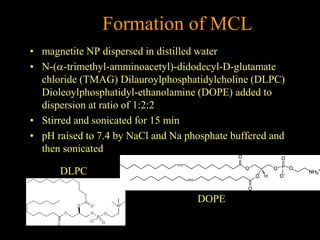
3. The formation methods of various NPs are described, including controlling size and coating to influence properties like plasma half-life. Cationic liposome coated magnetite NPs have also been investigated for their cell membrane interaction and uptake.