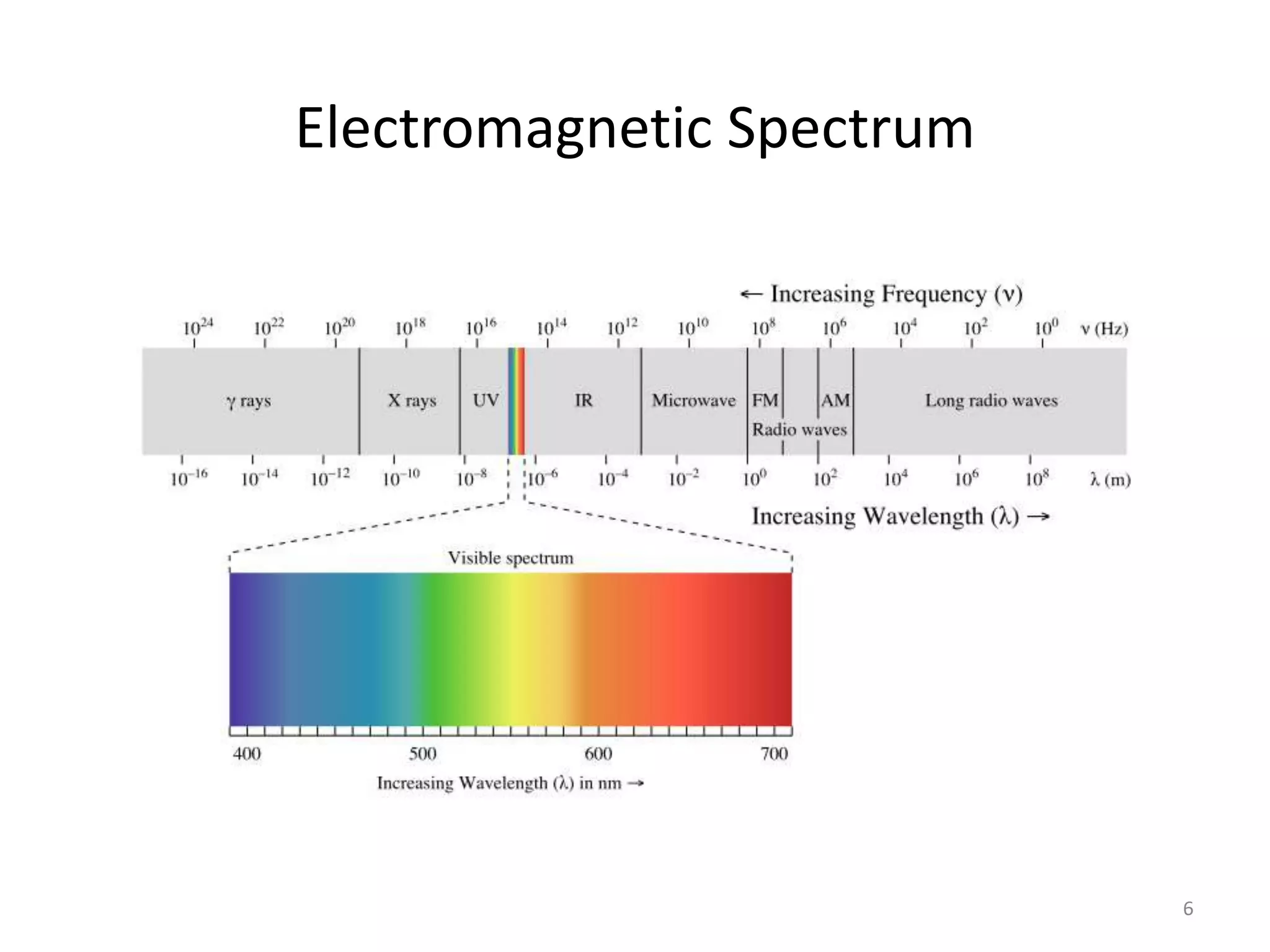
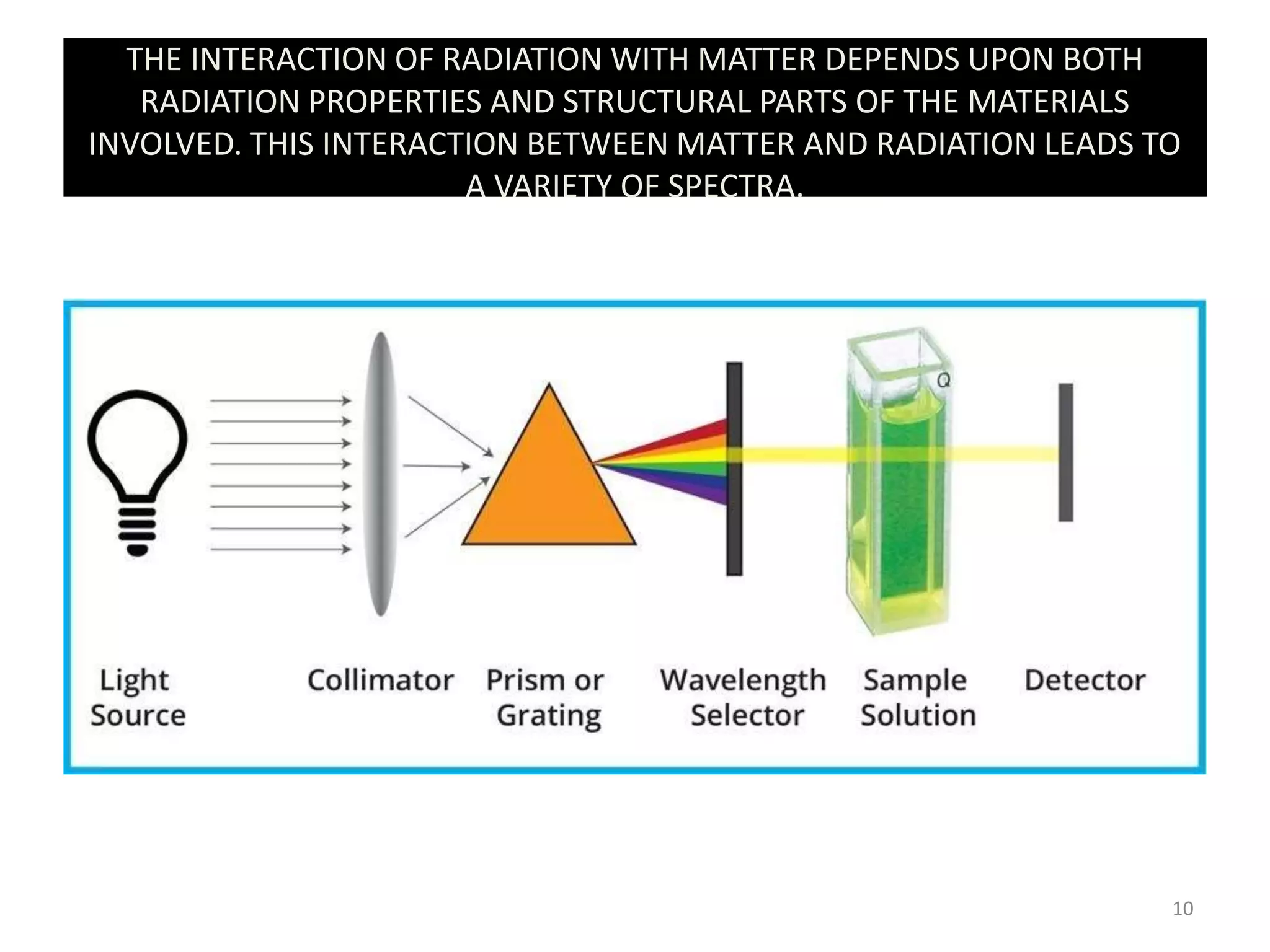
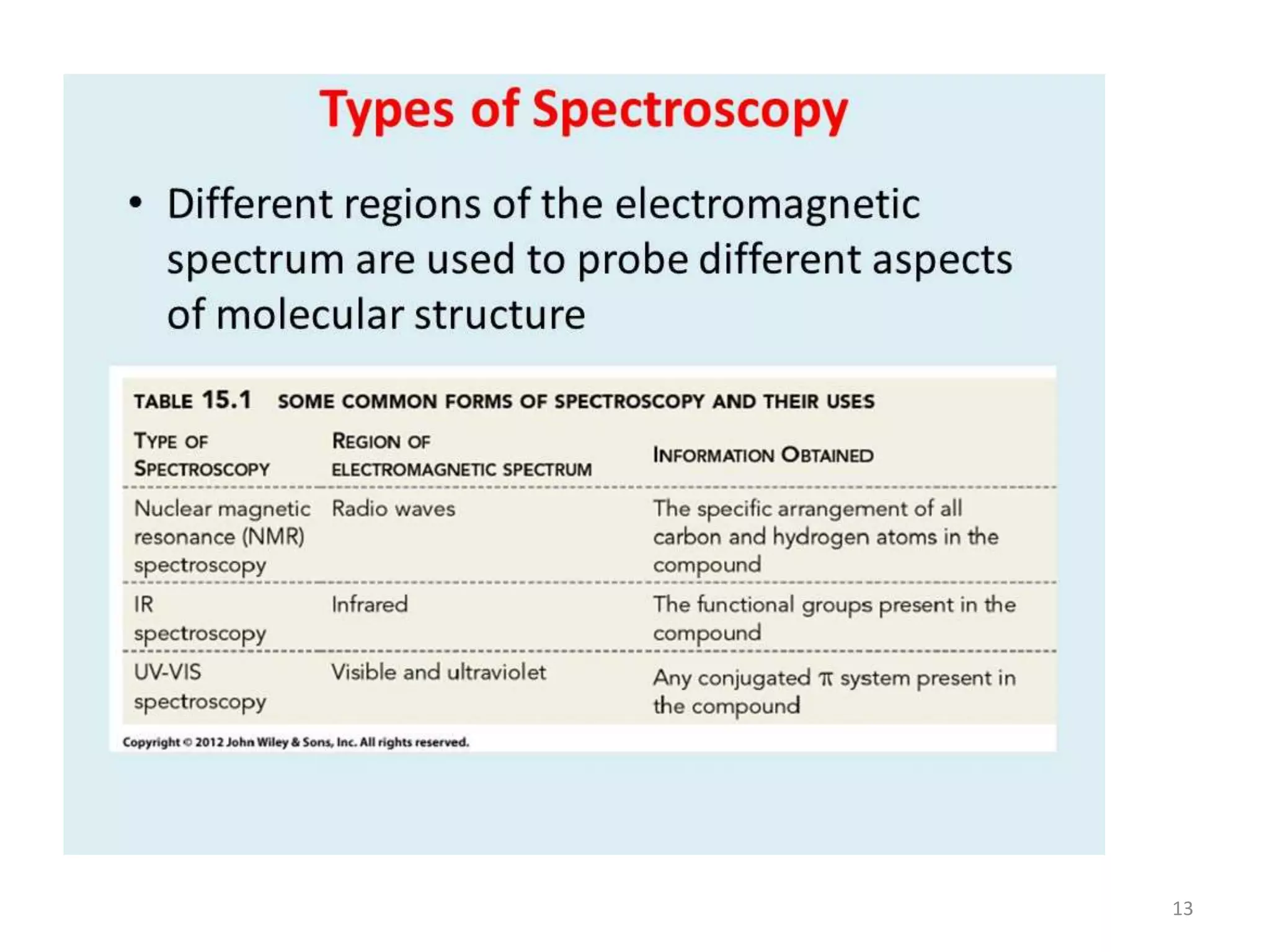
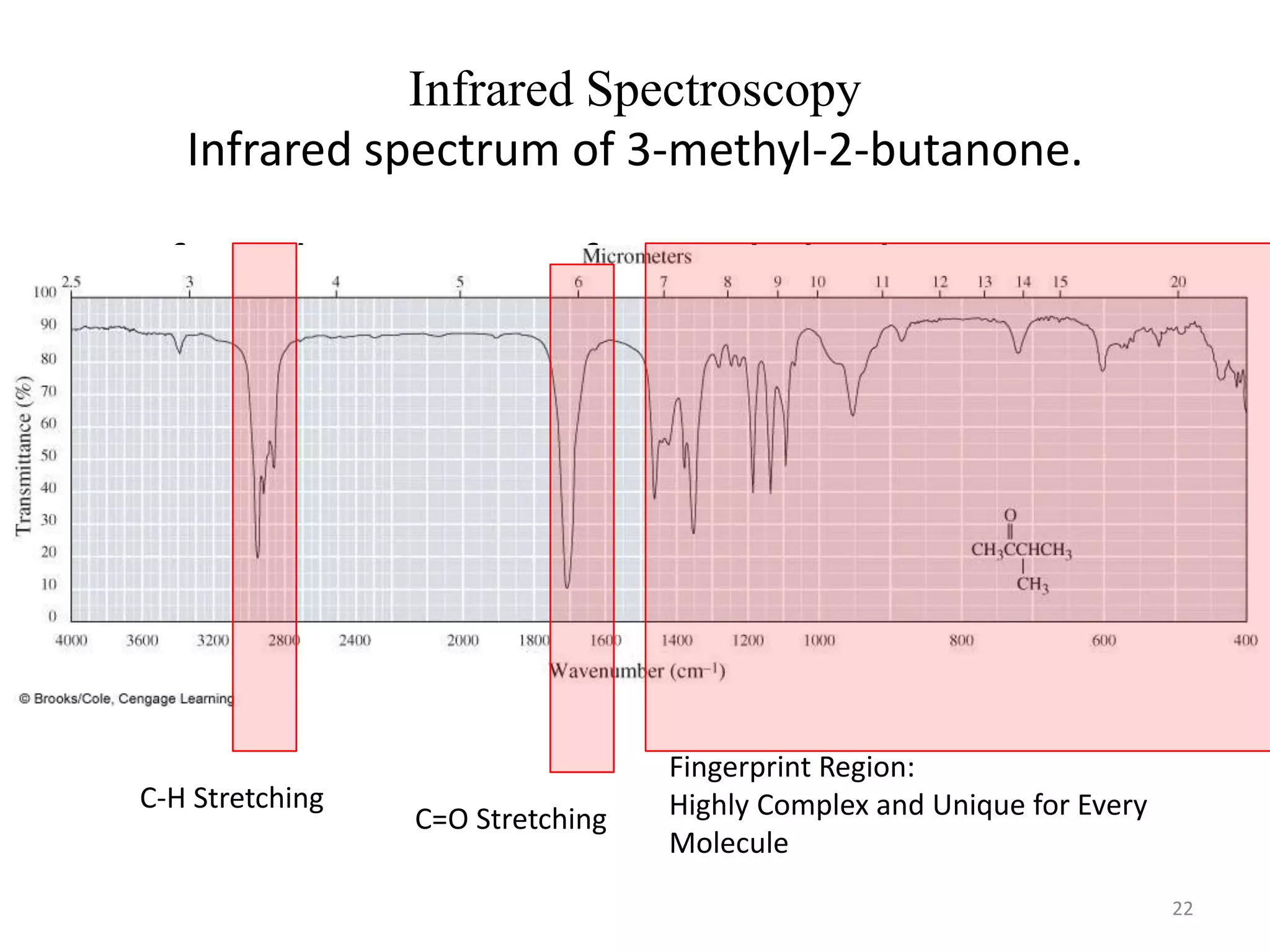
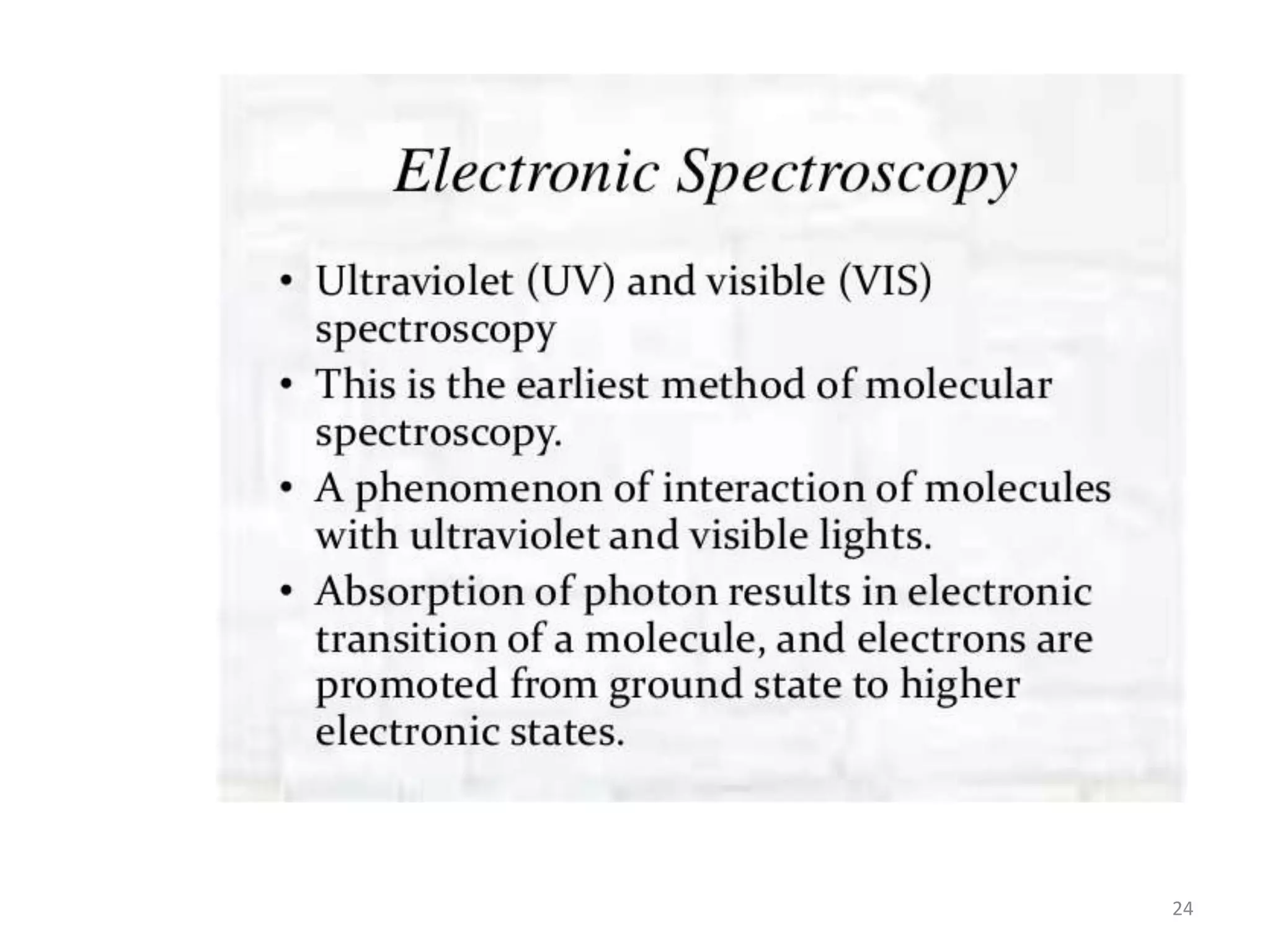
Spectroscopy deals with the interaction of electromagnetic radiation with matter. Different types of spectroscopy utilize different regions of the electromagnetic spectrum and provide information about molecular structure. Infrared spectroscopy analyzes vibrational and rotational transitions that occur when molecules absorb infrared radiation. It can be used to determine functional groups and molecular structure. Ultraviolet-visible spectroscopy analyzes electronic transitions that occur when molecules absorb ultraviolet or visible radiation. It provides information about molecular structure and composition.