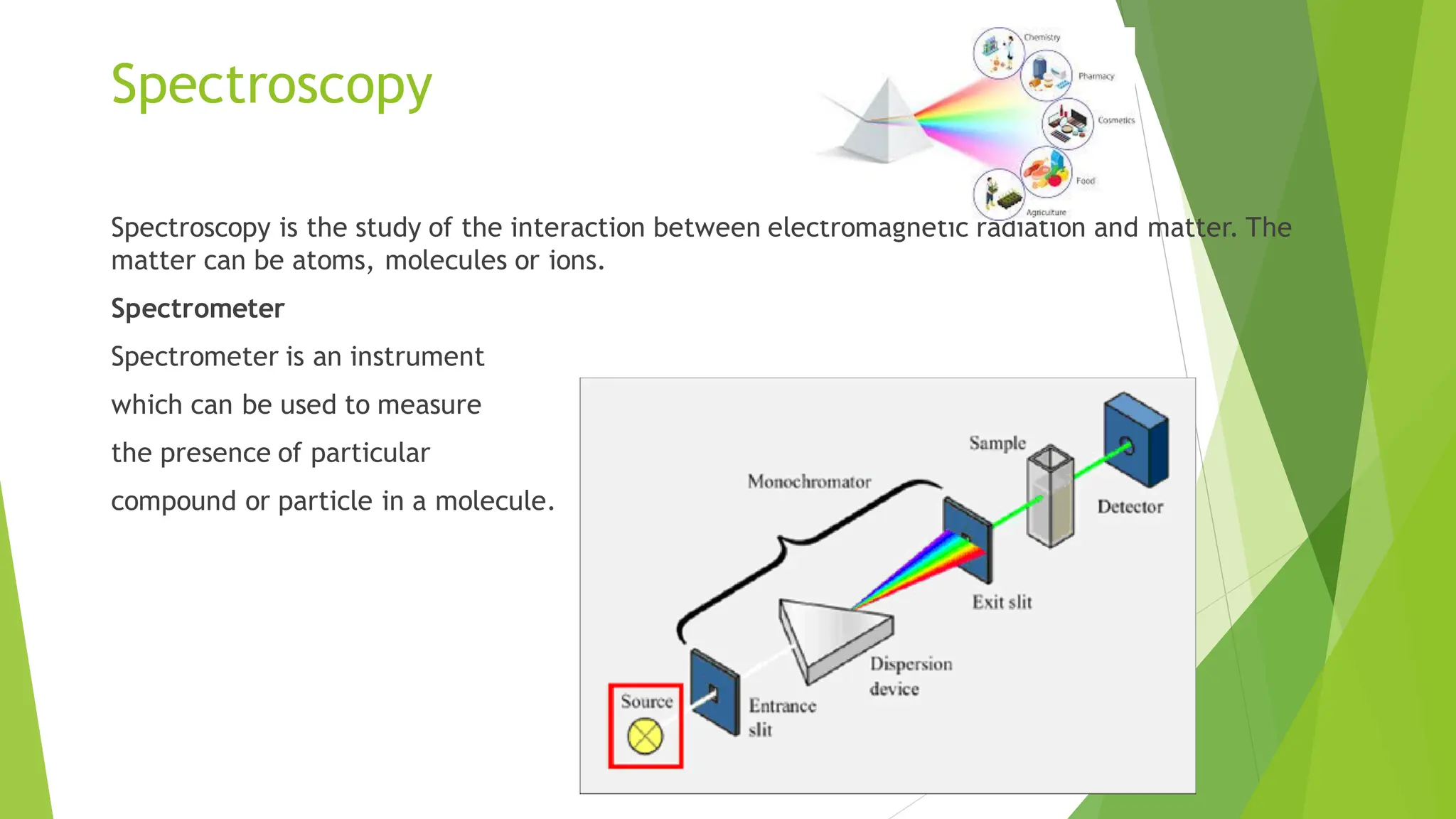
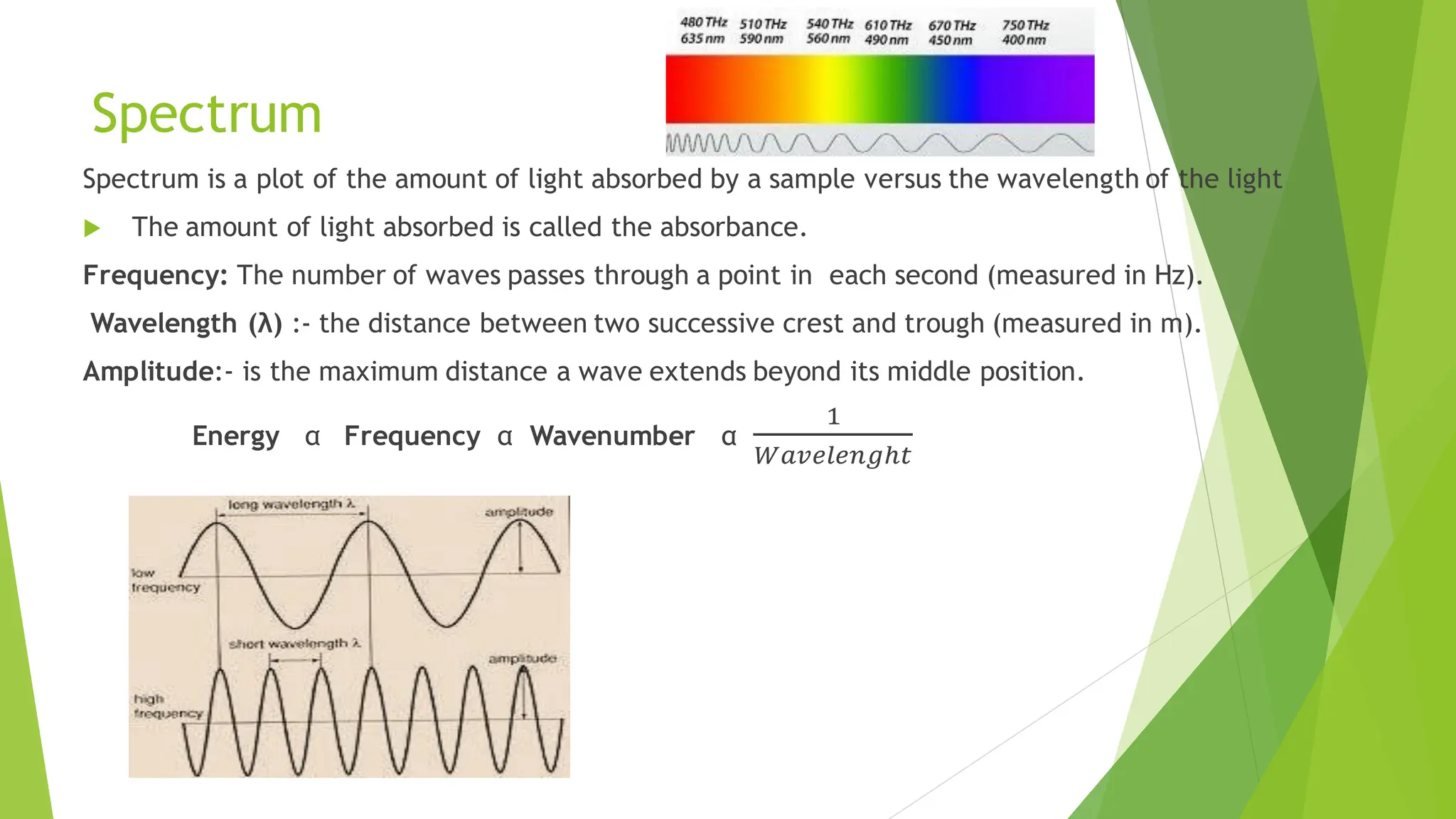
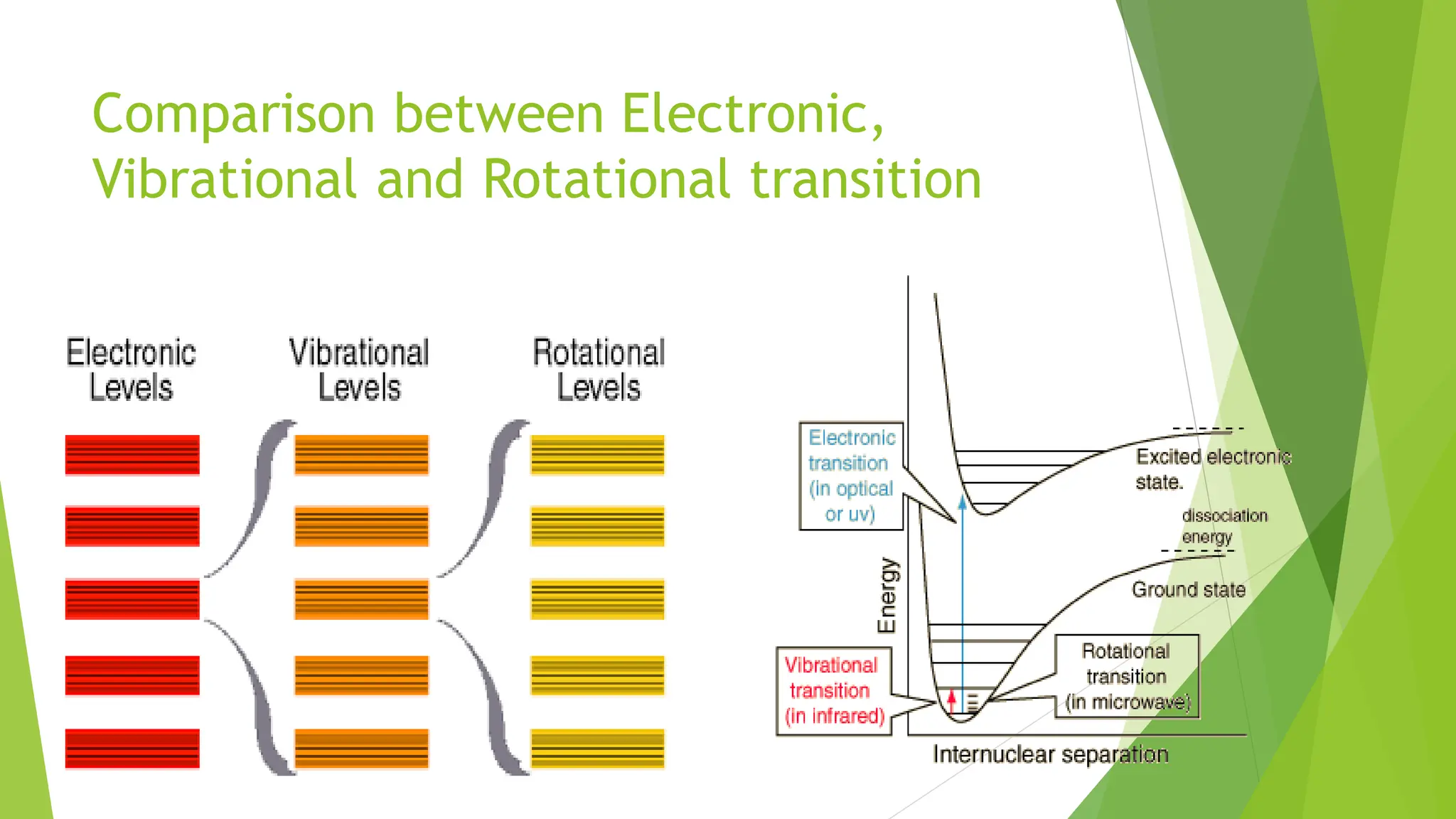
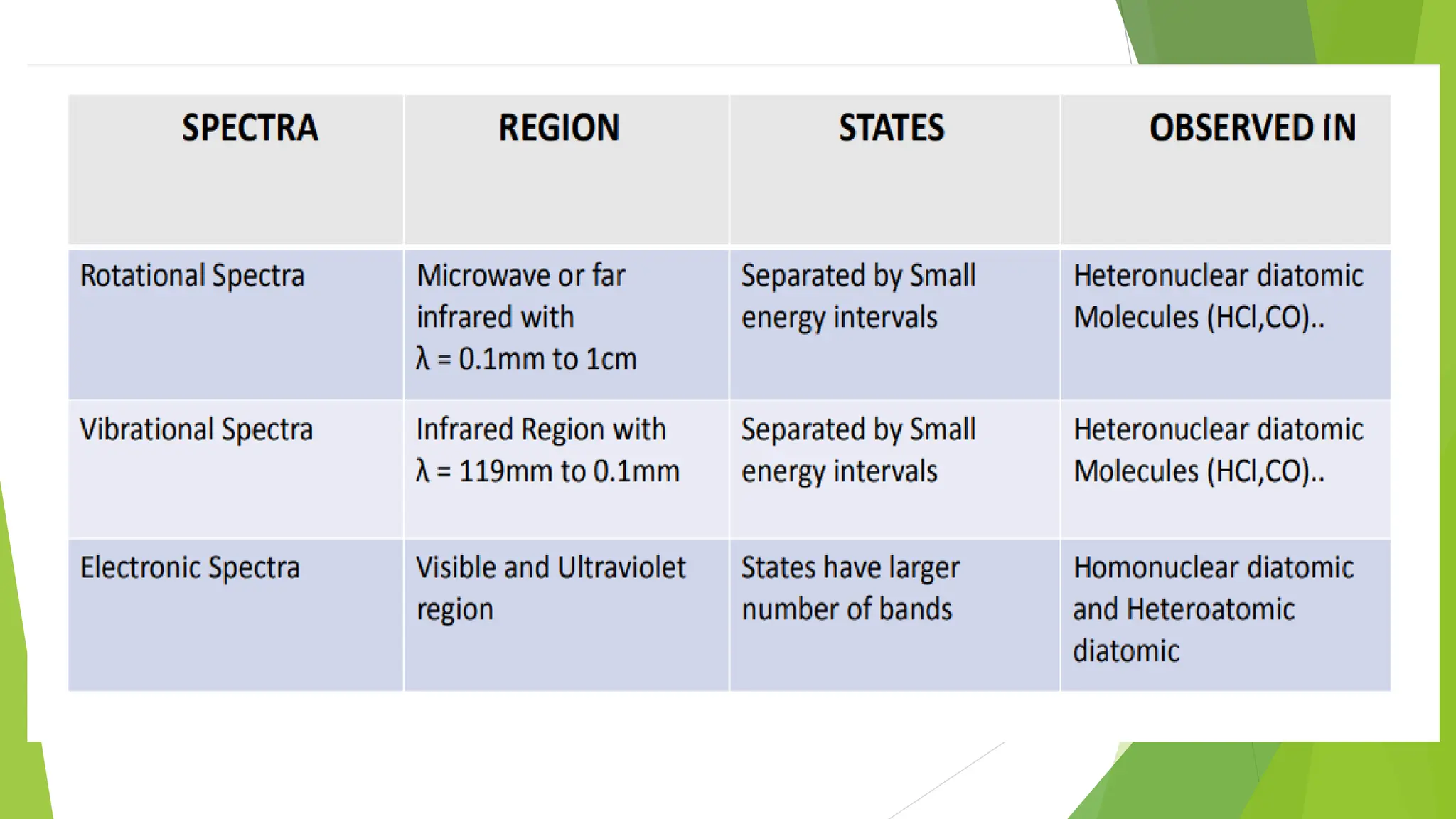
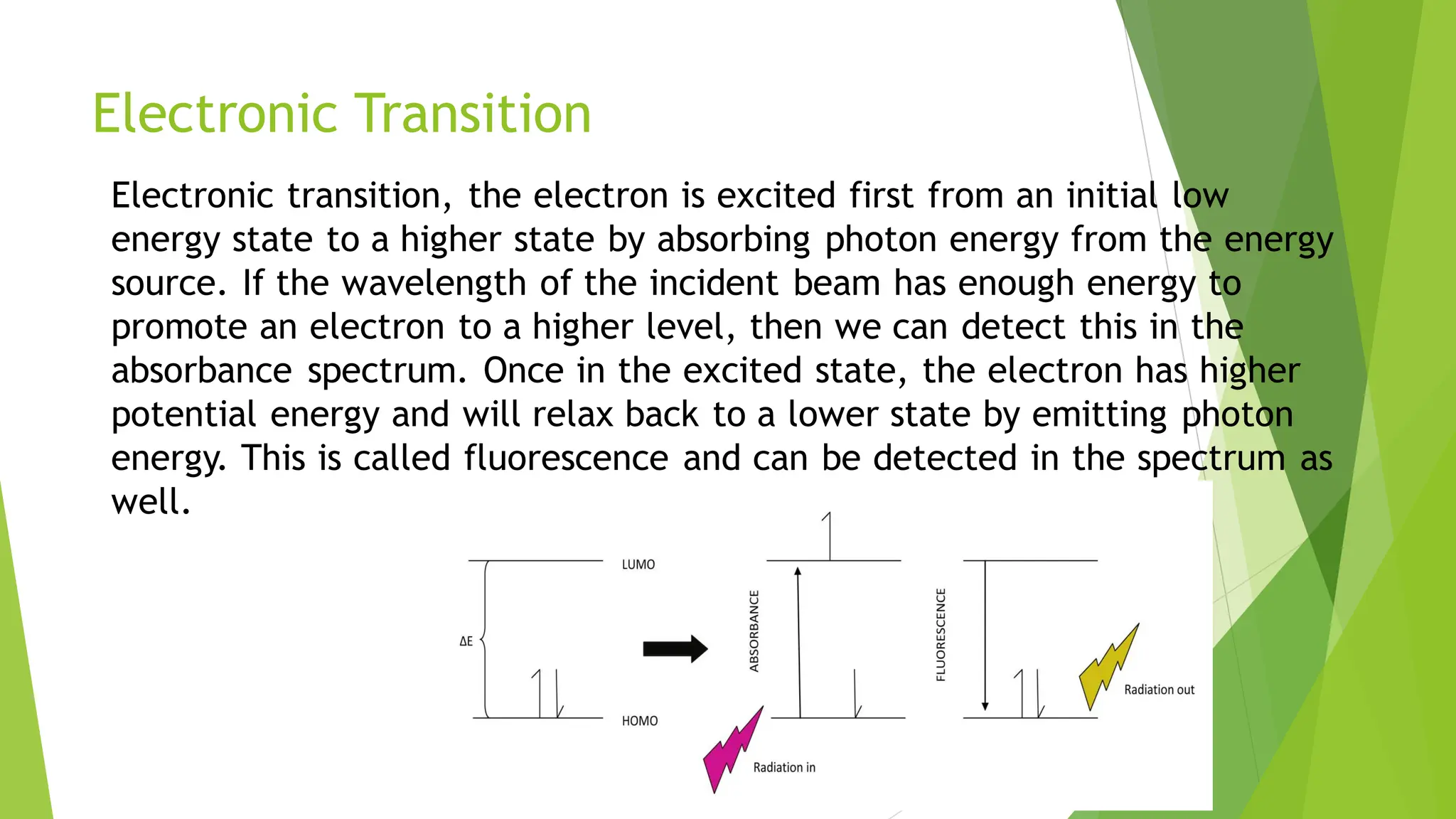
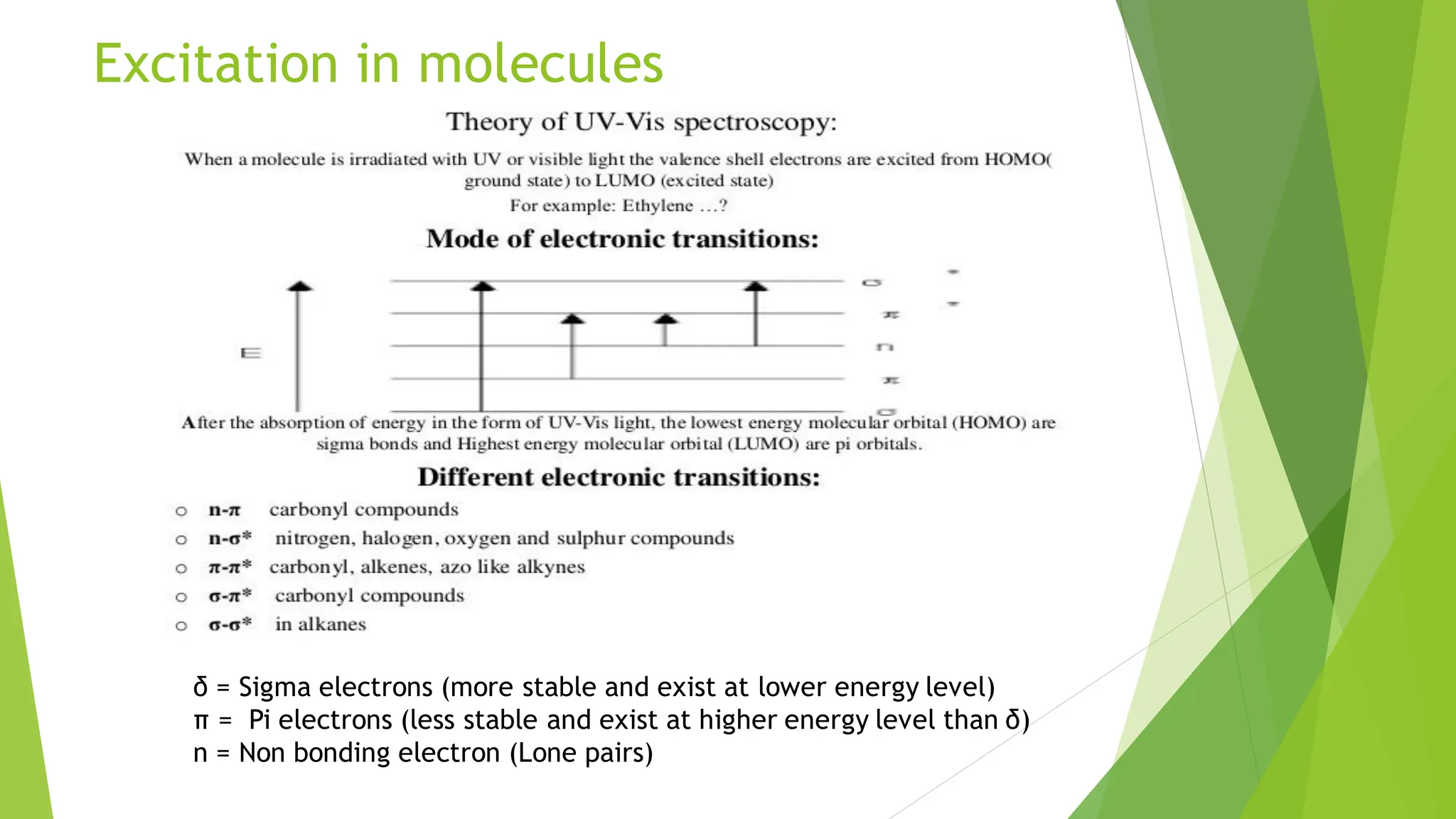
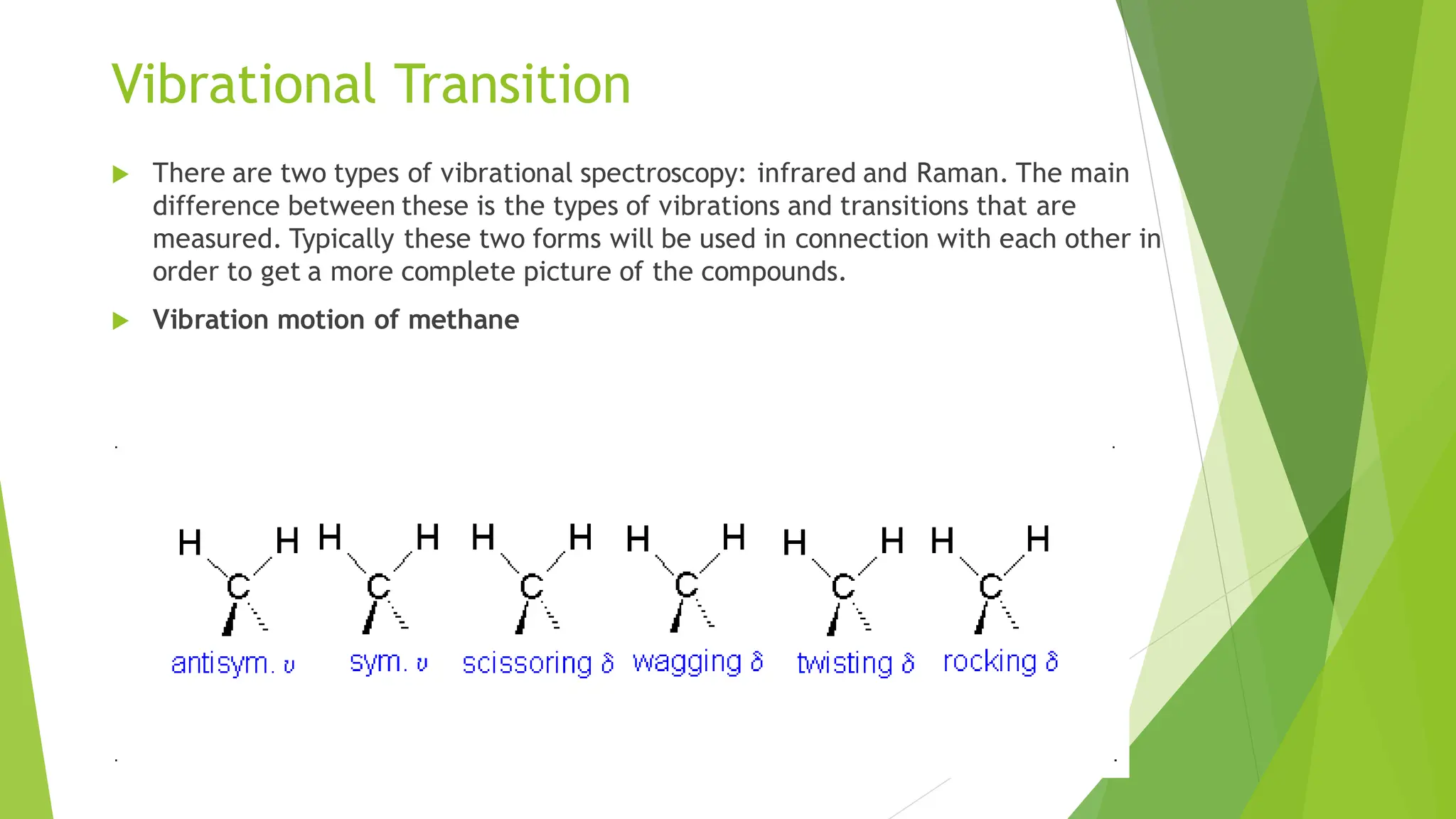
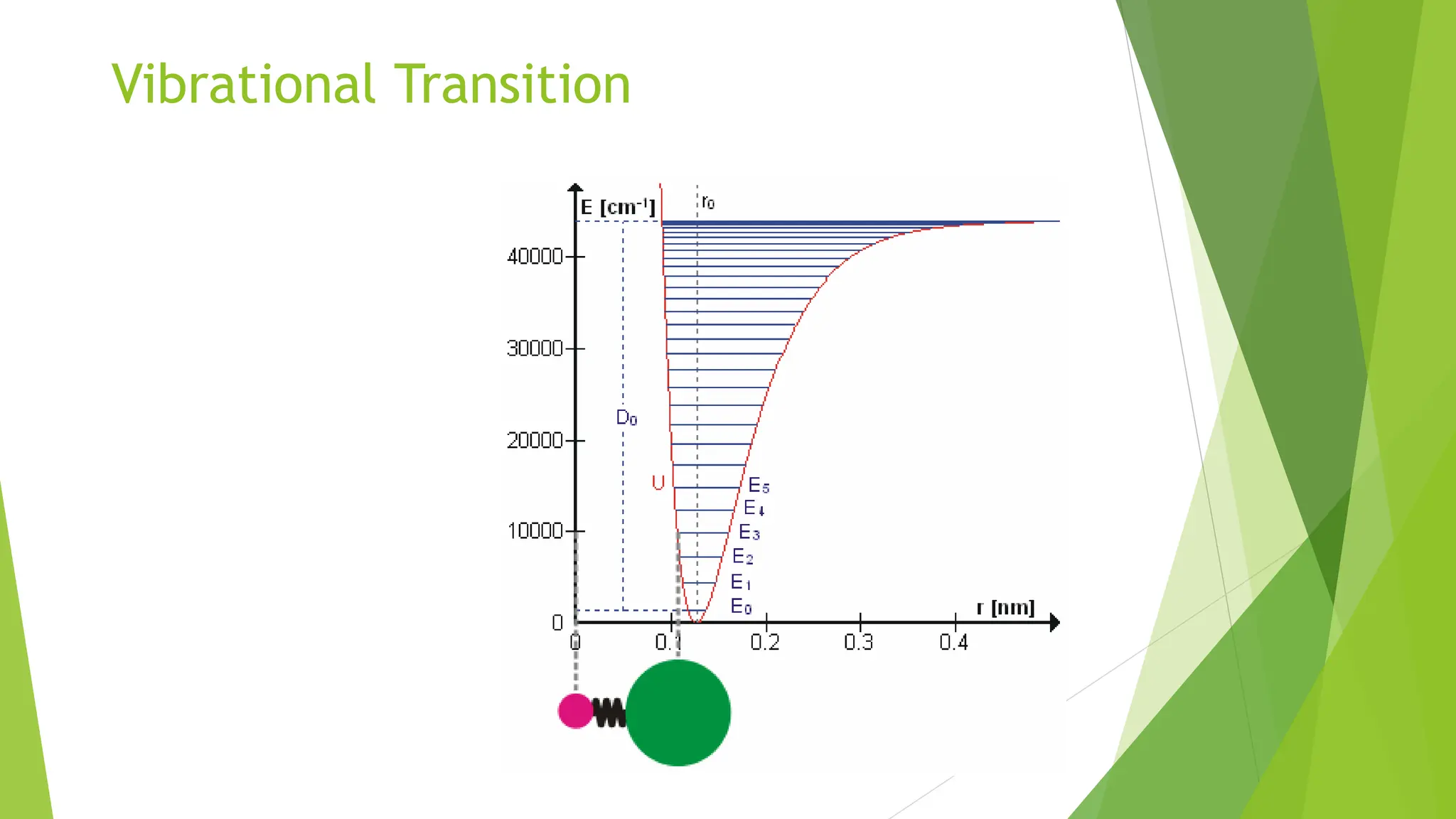
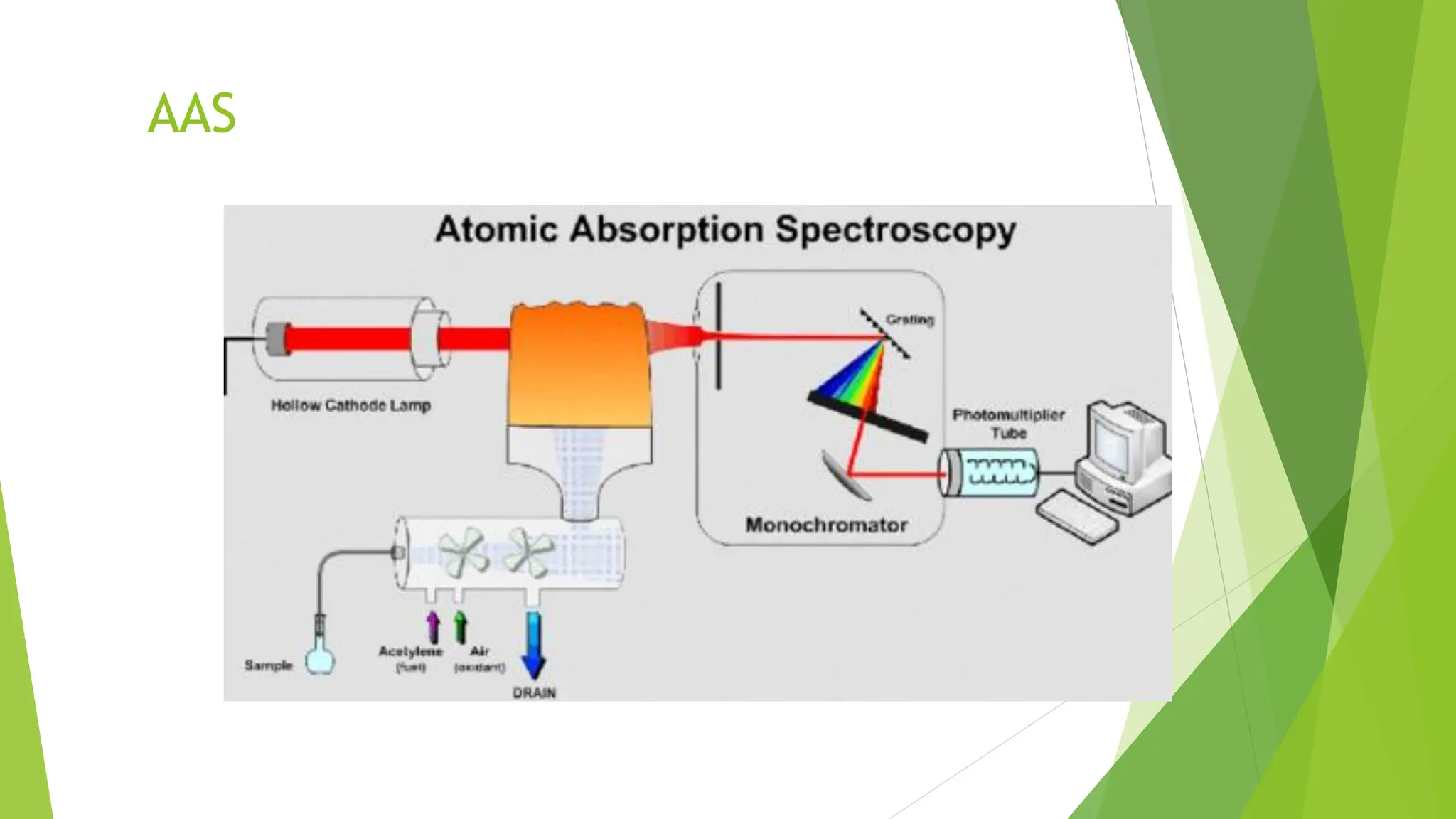
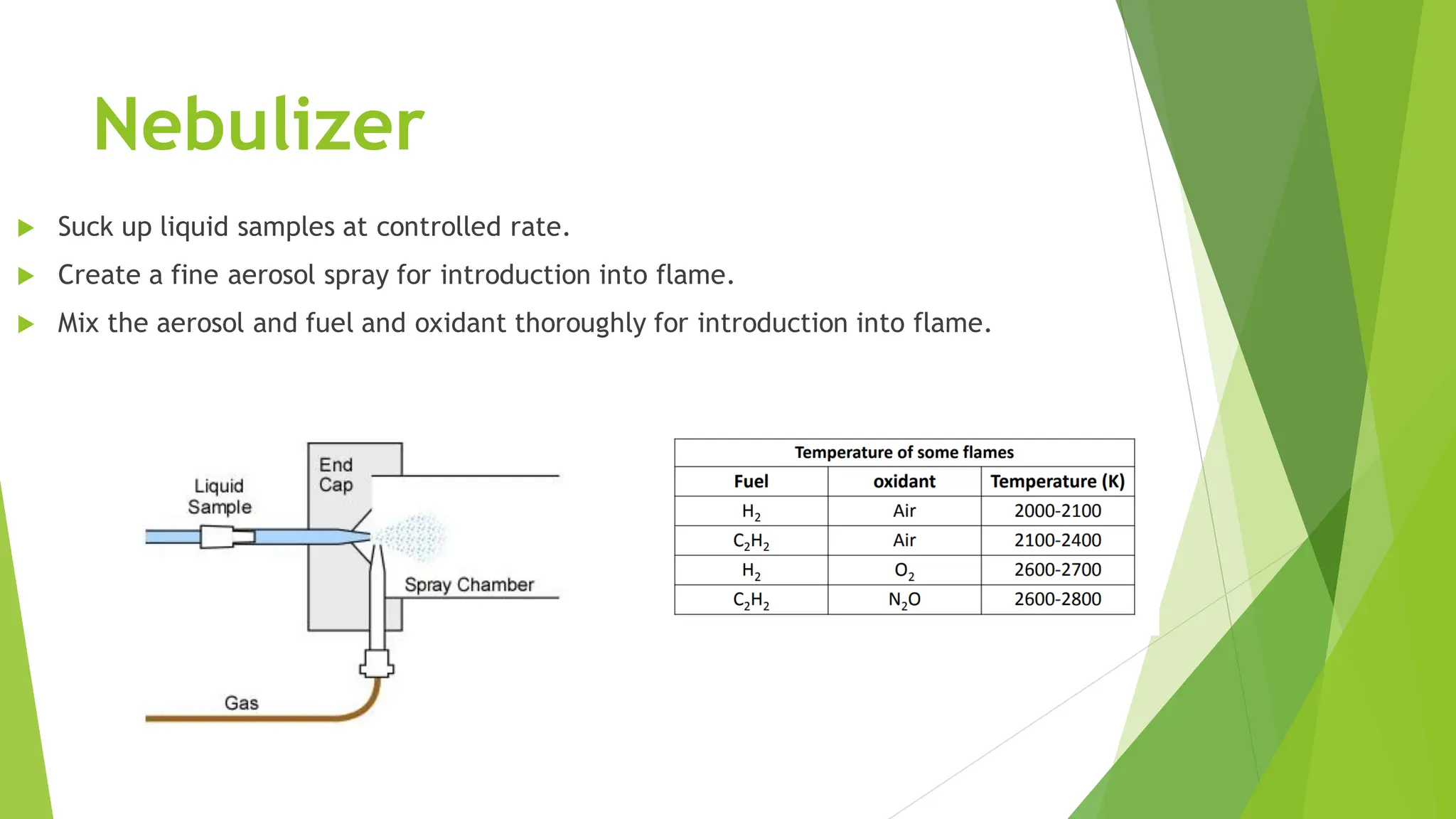
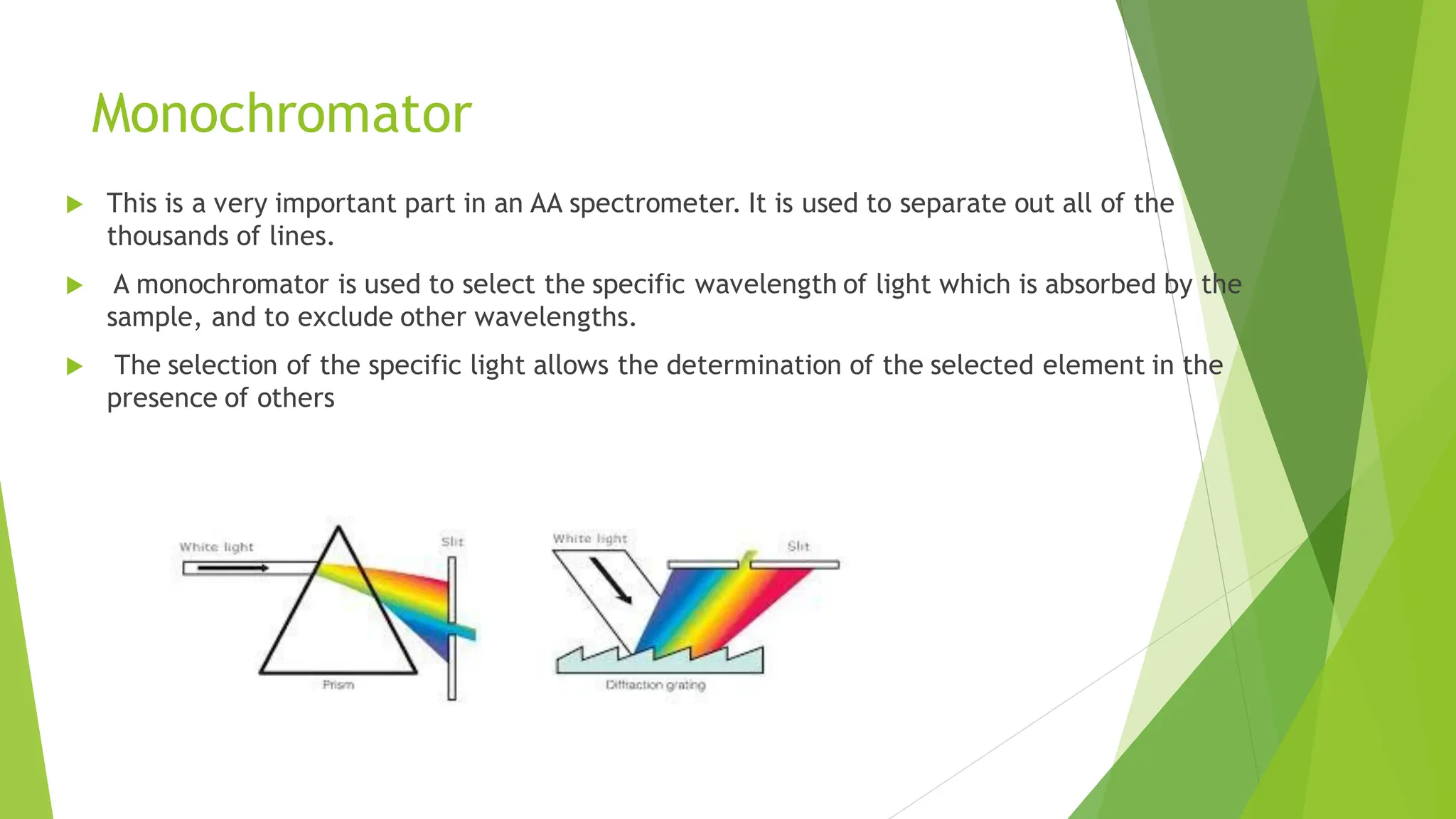
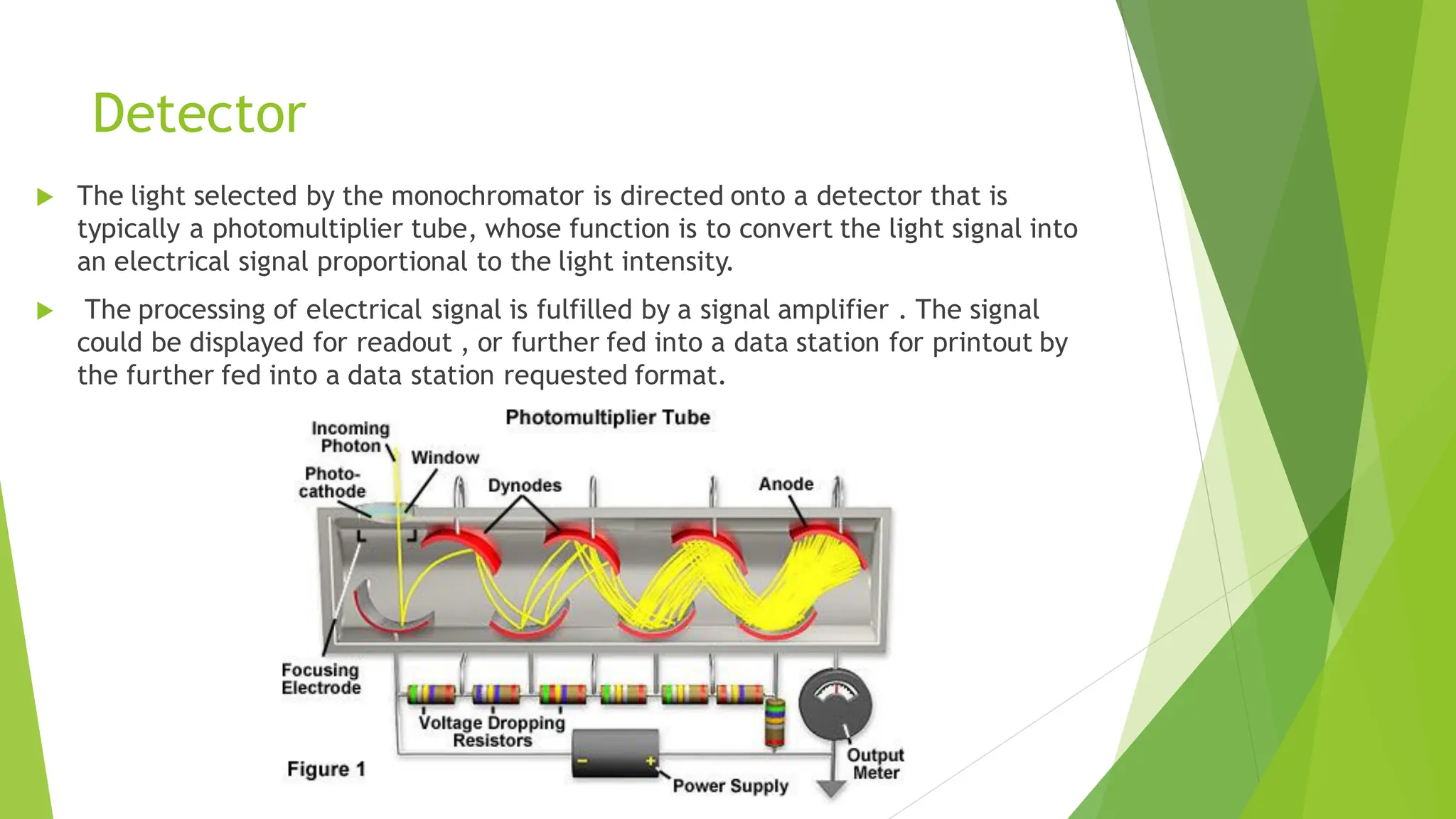
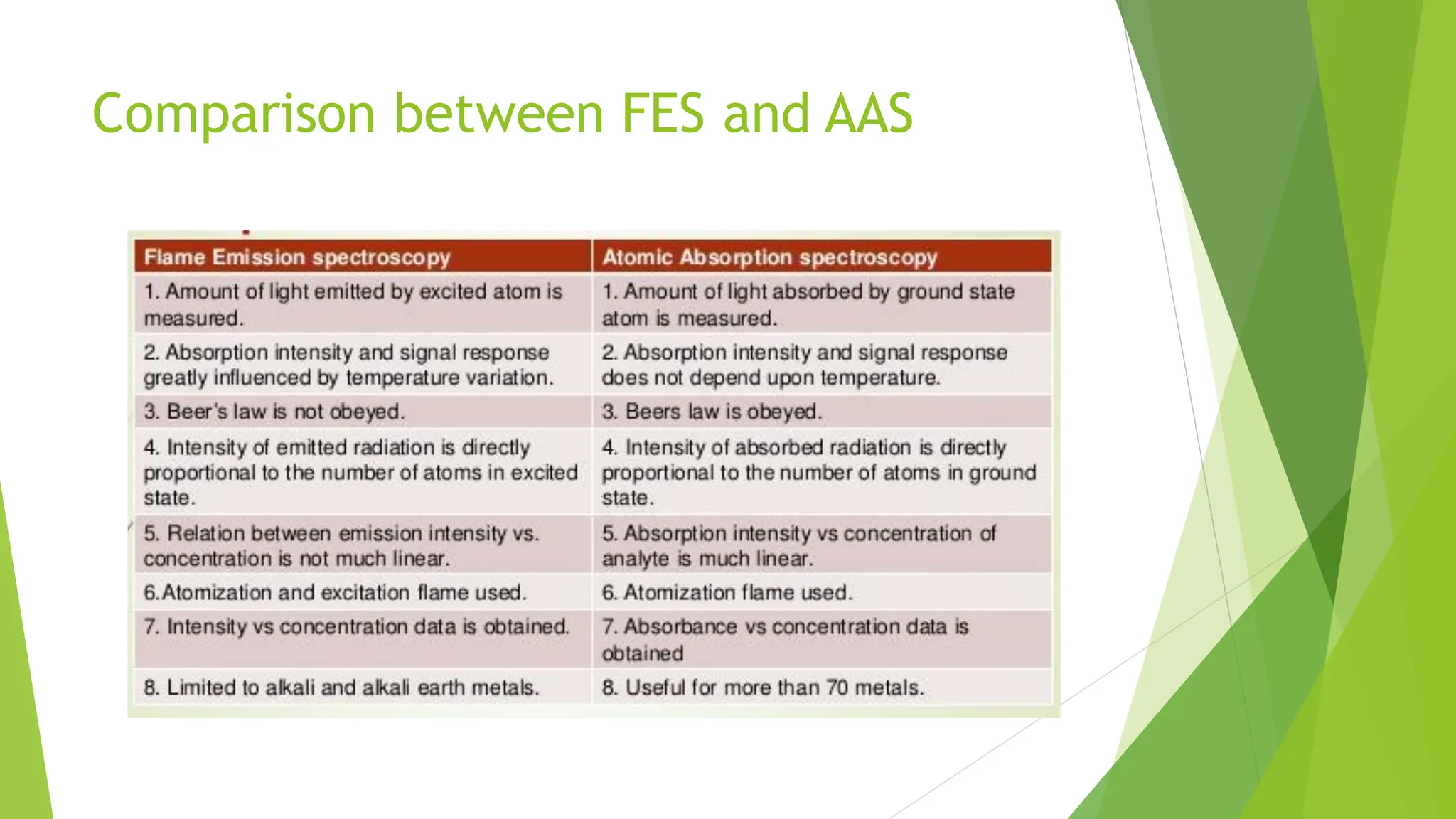
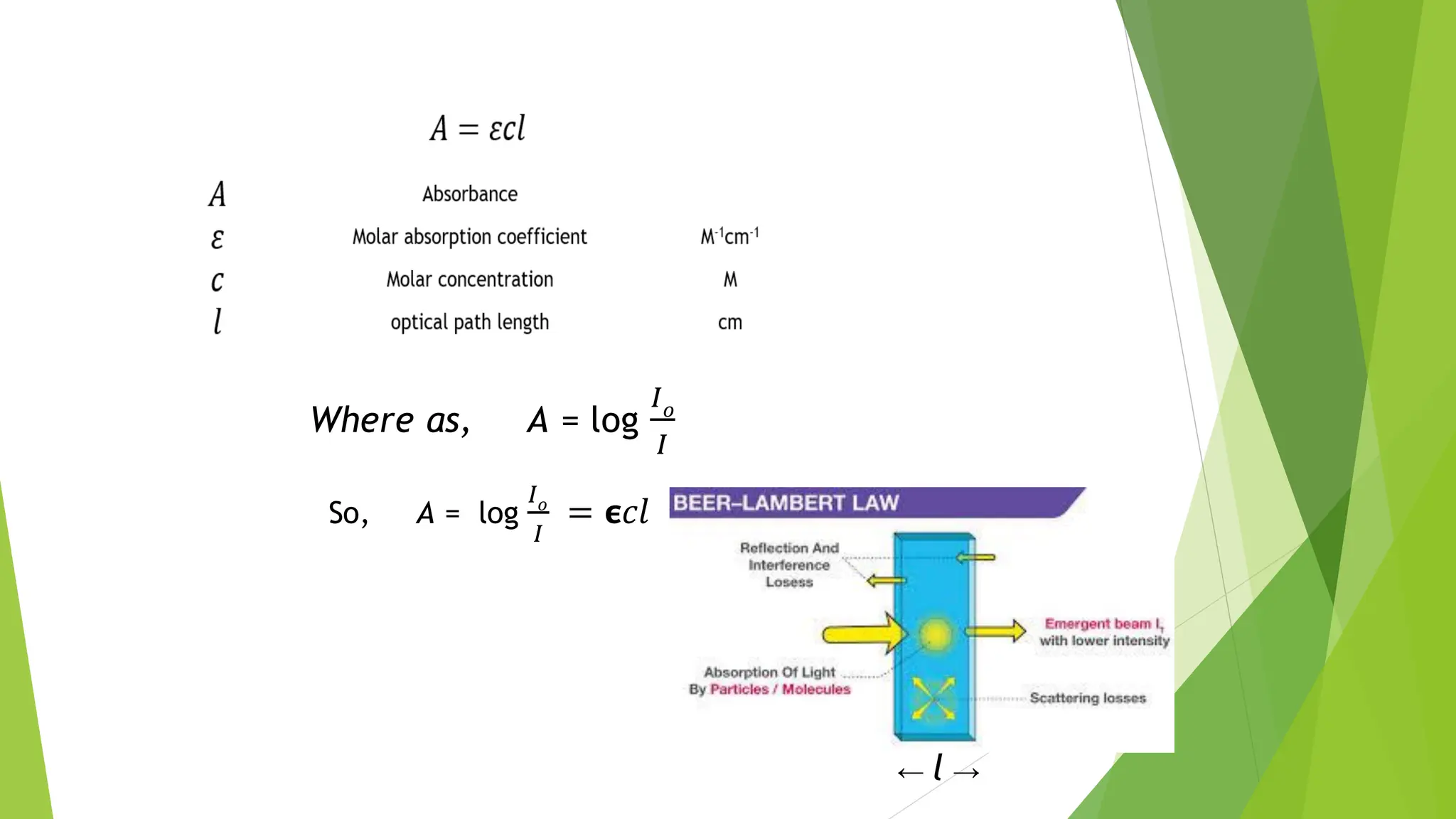
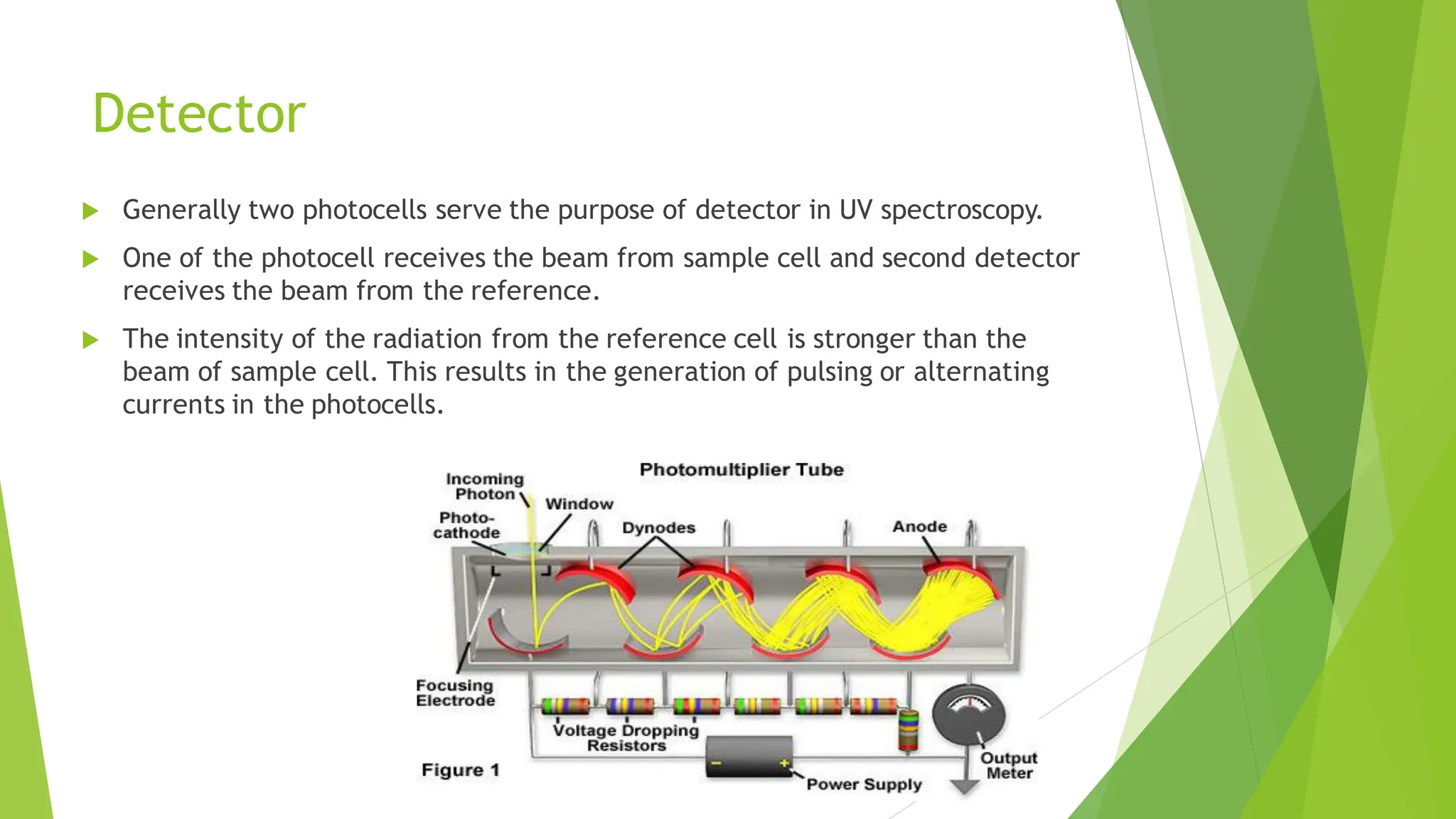
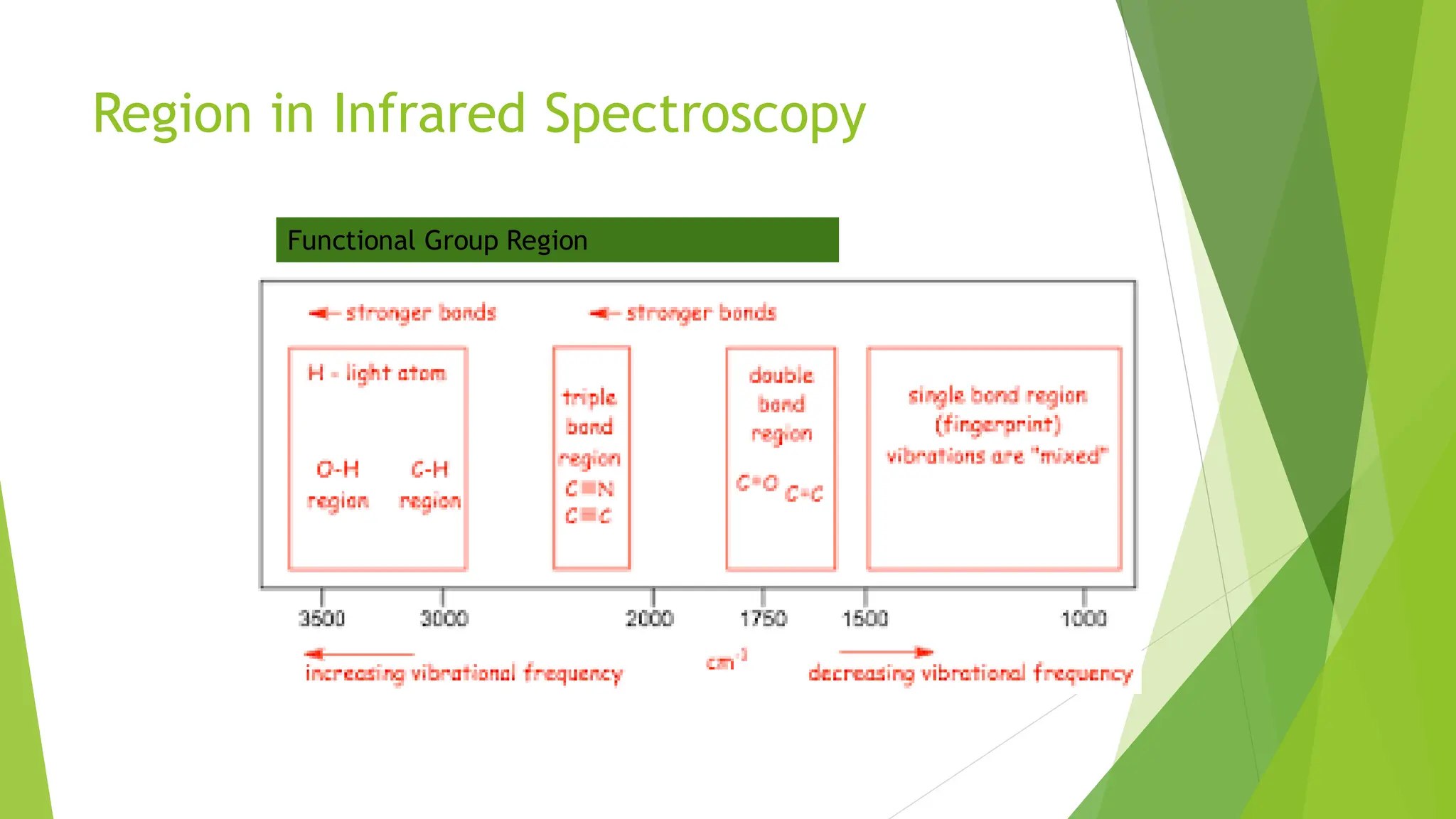
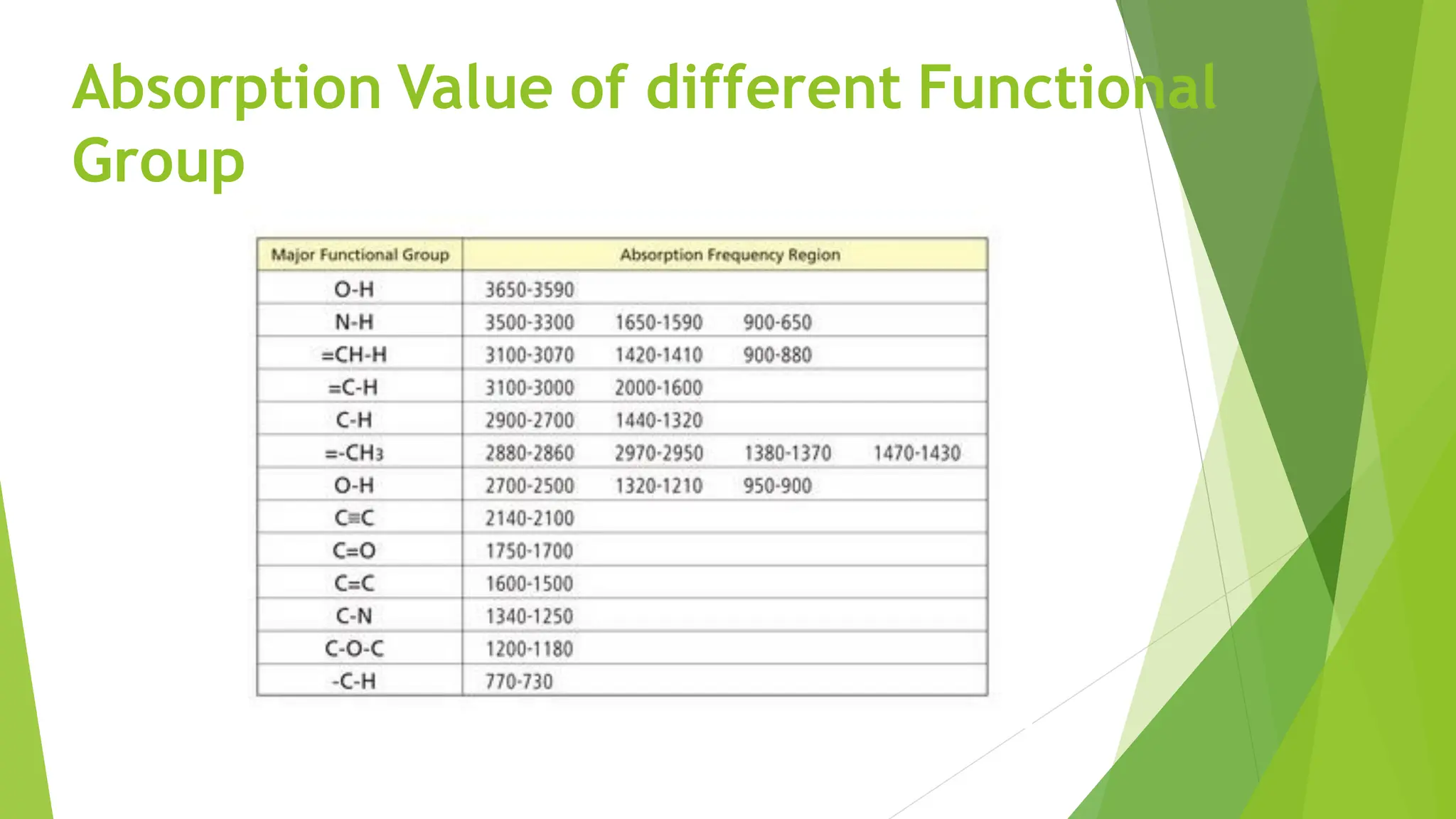
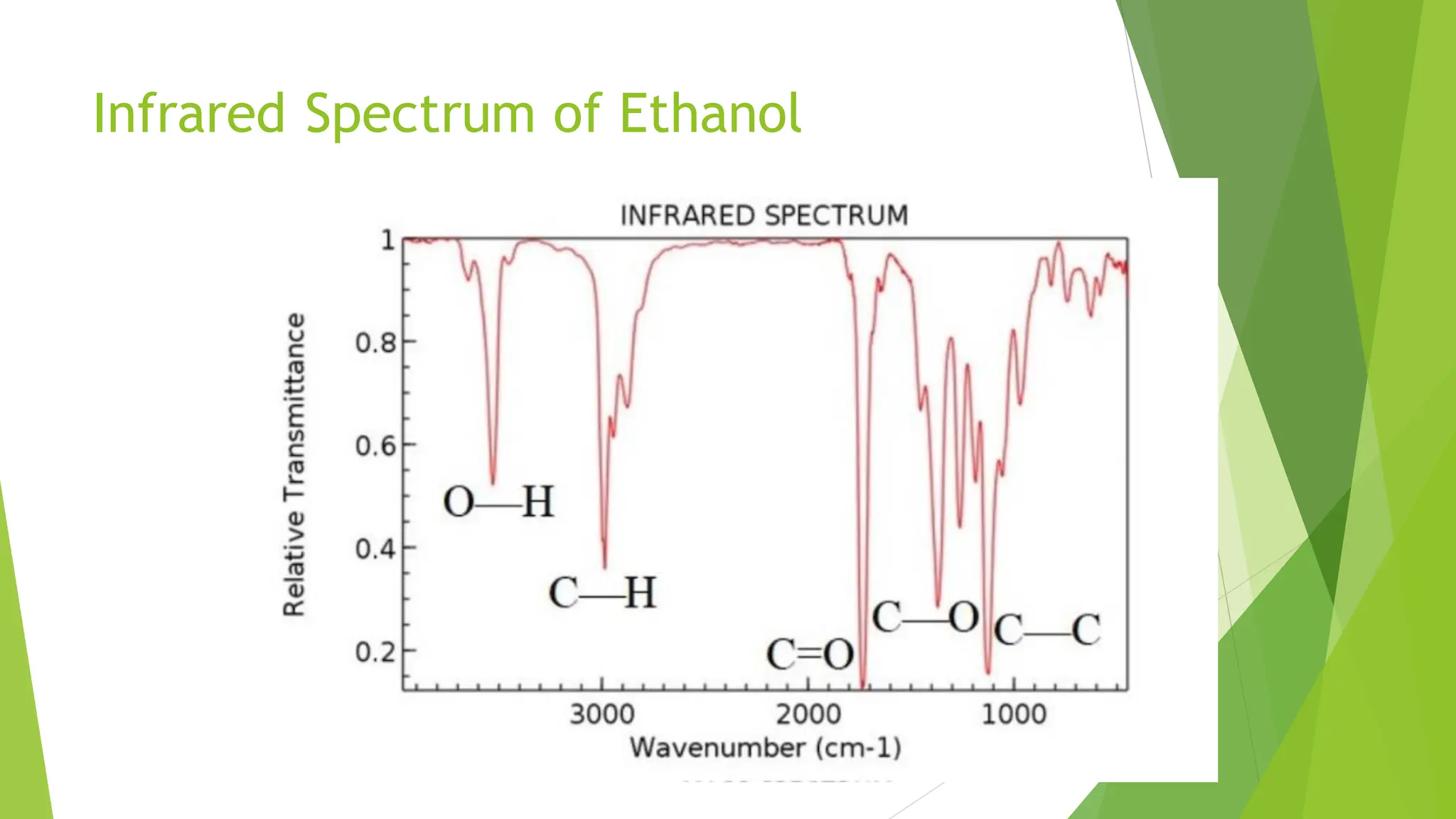
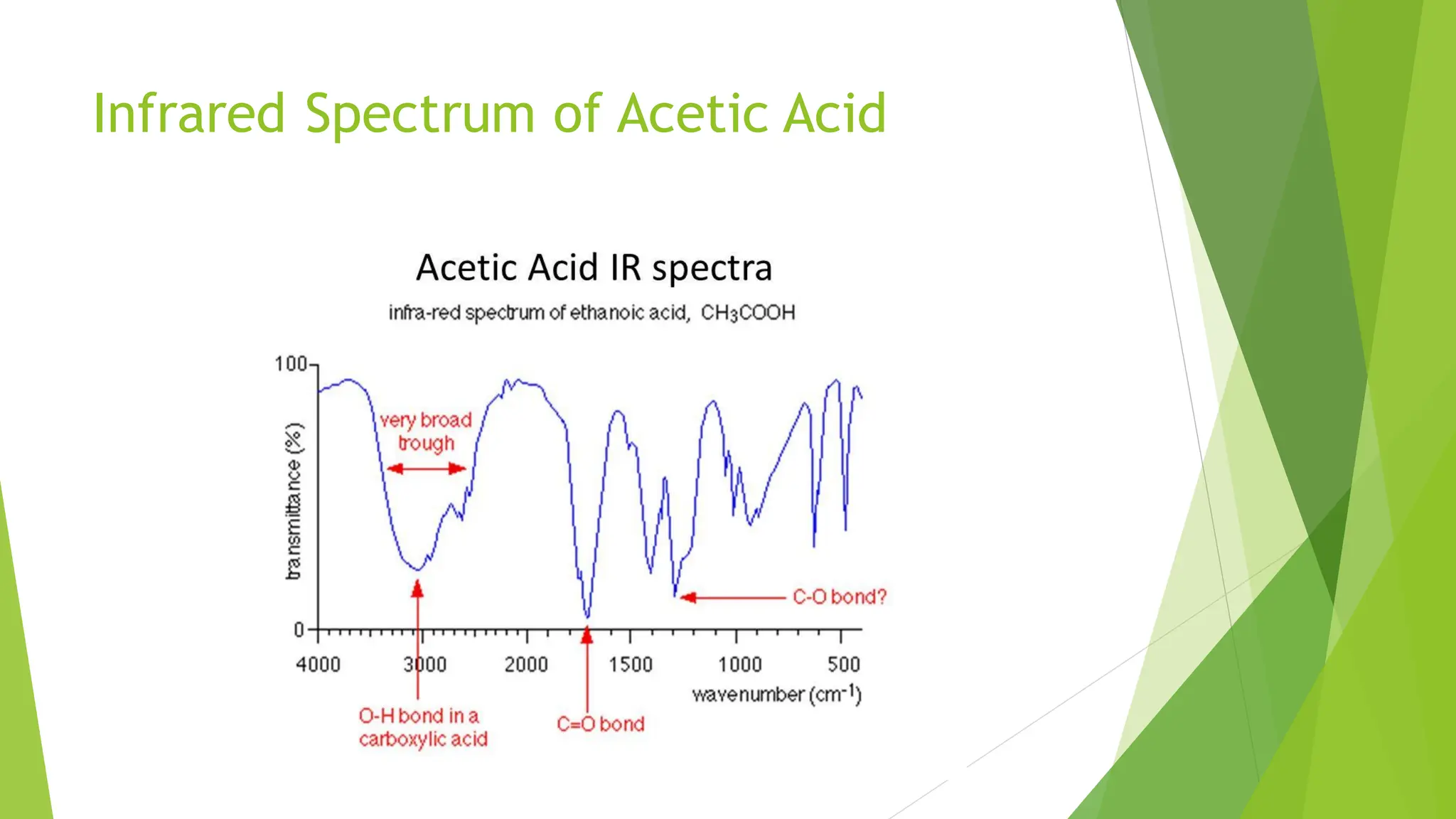
The document provides a comprehensive overview of spectroscopy, detailing the interaction of electromagnetic radiation with matter and the various types of spectroscopy techniques used for analyzing compounds. It covers fundamental principles, the role of different components in spectrometers, and applications of methods such as atomic absorption spectroscopy, infrared spectroscopy, and UV-visible spectroscopy. Additionally, it discusses the unique vibrational and electronic transitions of molecules that help identify and characterize different compounds.