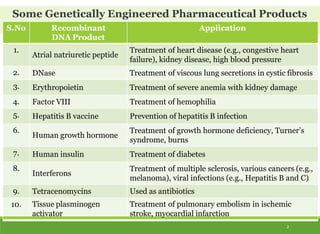
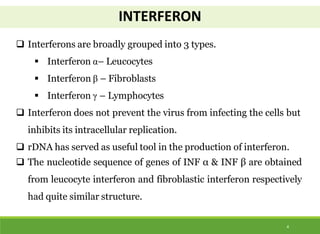
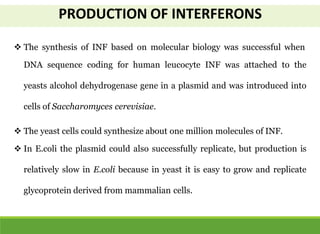
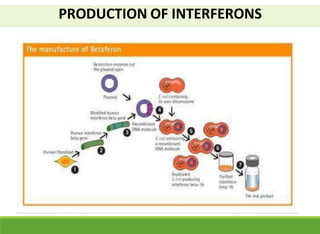
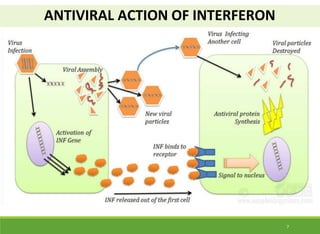
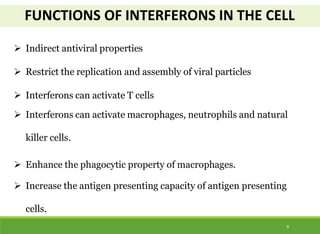
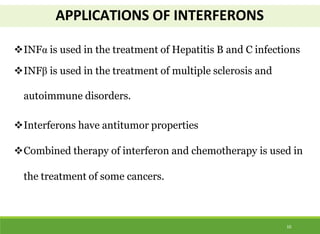
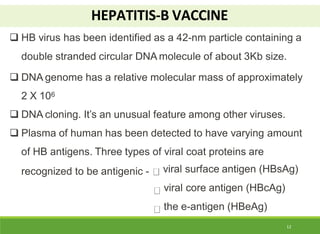
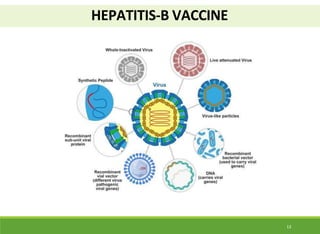
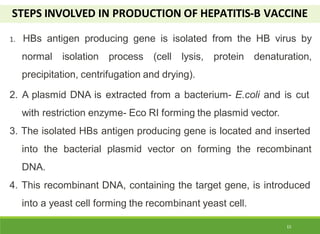
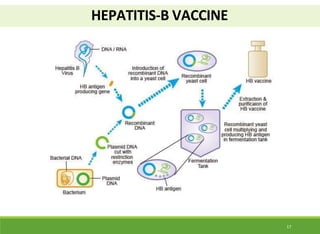
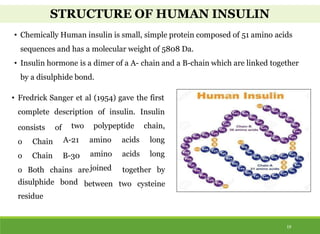
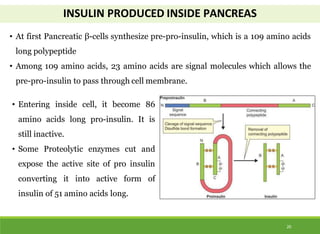
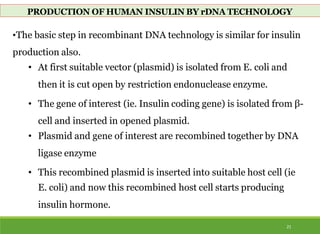
This document discusses the application of recombinant DNA technology in producing important pharmaceutical products like interferon, hepatitis B vaccine, and human insulin. It provides details on how interferon is produced by introducing the interferon gene into yeast or E. coli cells. The hepatitis B vaccine is produced by isolating the hepatitis B surface antigen gene and inserting it into plasmid DNA in bacteria to produce the antigen. Finally, human insulin was one of the earliest products produced using recombinant DNA technology by isolating the insulin gene and expressing it in E. coli cells to synthesize insulin protein.