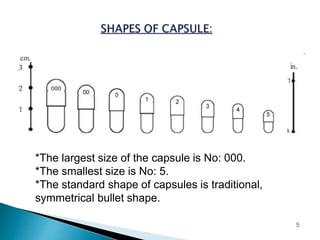
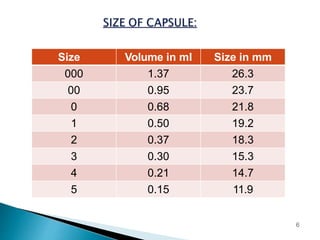
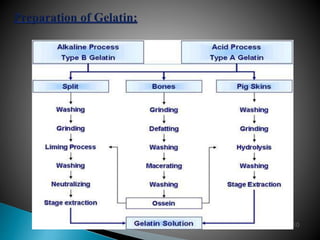
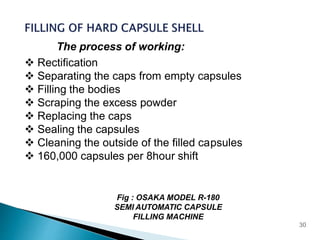
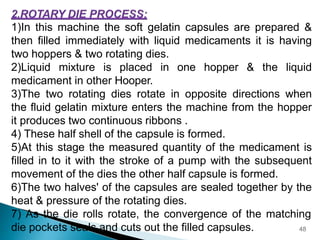
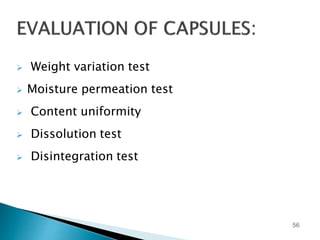
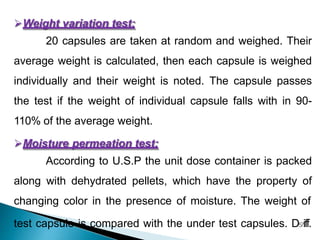
Hard and soft gelatin capsules are solid oral dosage forms where the drug is enclosed within a soluble shell made of gelatin. Hard gelatin capsules have a two-piece design where the body and cap are joined, while soft gelatin capsules are one-piece shells containing liquids or semisolids. Some key advantages of capsules are taste masking, ease of administration and manufacture, and ability to incorporate both solid and liquid drugs. The document discusses the history, components, manufacturing processes, sizes, and storage conditions of hard and soft gelatin capsules.