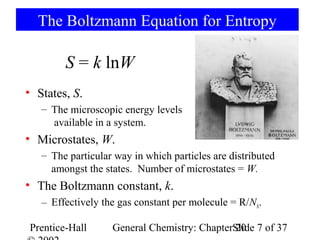
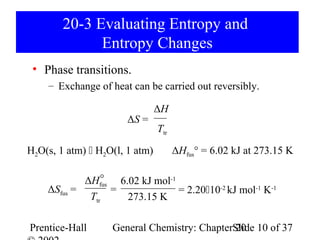
This document summarizes Chapter 20 from the textbook "General Chemistry: Principles and Modern Applications" by Petrucci, Harwood, and Herring. The chapter discusses spontaneous change, entropy, free energy, and criteria for spontaneity. It defines key concepts like entropy, the second law of thermodynamics, standard free energy change, and how free energy relates to chemical equilibrium. The chapter also examines how these principles apply to temperature dependence of equilibrium constants and coupled chemical reactions.









![Absolute Entropies
• Third law of thermodynamics.
– The entropy of a pure perfect crystal at 0 K is zero.
• Standard molar entropy.
– Tabulated in Appendix D.
ΔS = [ νpS°(products) - νrS°(reactants)]
Prentice-Hall General Chemistry: ChapterSlide 13 of 37
20](https://image.slidesharecdn.com/ch20-130105201707-phpapp02/85/Ch20-13-320.jpg)





![20-5 Standard Free Energy Change, ΔG°
• The standard free energy of formation, ΔGf°.
– The free energy change for a reaction in which a
substance in its standard state is formed from its
elements in reference forms in their standard states.
• The standard free energy of reaction, ΔG°.
ΔG° = [ νp ΔGf°(products) - νr ΔGf°(reactants)]
Prentice-Hall General Chemistry: ChapterSlide 21 of 37
20](https://image.slidesharecdn.com/ch20-130105201707-phpapp02/85/Ch20-21-320.jpg)
















![Focus On Coupled Reactions in
Biological Systems
ADP3- + HPO42- + H+ → ATP4- + H2O
ΔG° = -9.2 kJ mol-1
aATPaH2O
ΔG = ΔG° + RT ln
aADPaPiaH3O+
But [H3O+] = 10-7 M not 1.0 M.
ΔG = -9.2 kJ mol-1 + 41.6 kJ mol-1
The biological standard state: = +32.4 kJ mol-1 = ΔG°'
Prentice-Hall General Chemistry: ChapterSlide 38 of 37
20](https://image.slidesharecdn.com/ch20-130105201707-phpapp02/85/Ch20-38-320.jpg)

