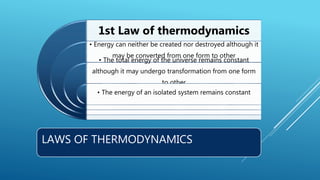
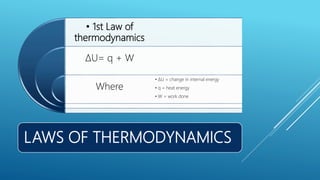
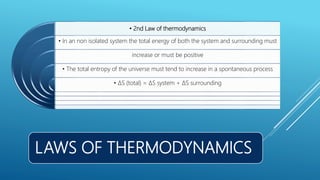
This document provides an overview of thermodynamics concepts including:
- Thermodynamics deals with energy changes in physical and chemical processes.
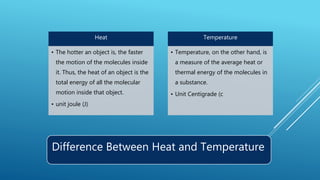
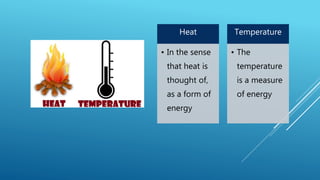
- Heat is a form of energy while temperature is a measure of average heat or thermal energy.
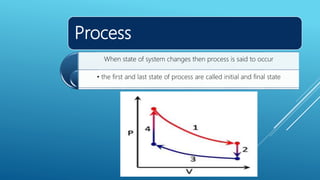
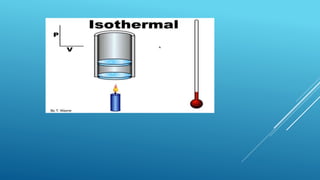
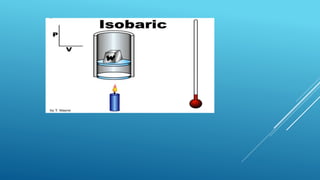
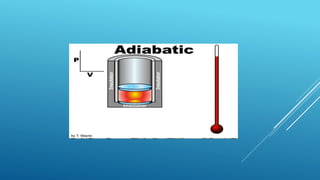
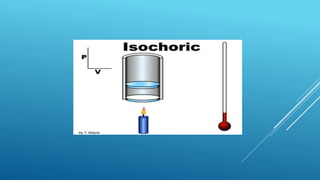
- Processes involve a change in state, such as isothermal, isobaric, adiabatic, and isochoric processes.
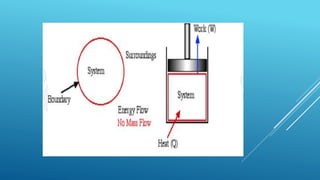
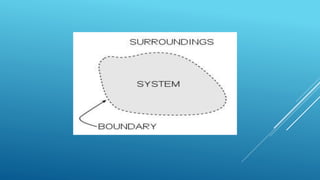
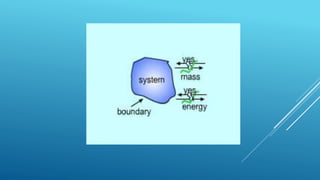
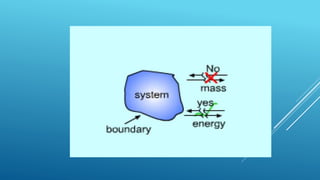
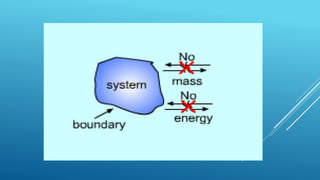
- A system exchanges energy but not mass with its surroundings in a closed system. An isolated system exchanges neither energy nor mass.