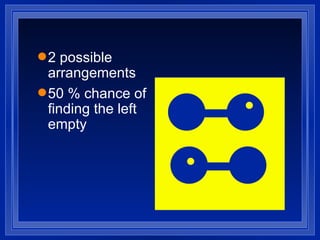
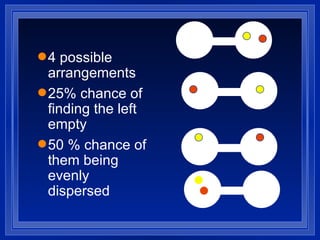
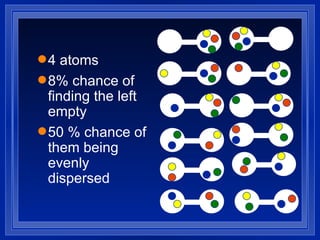
1. Entropy is a measure of disorder in a system. According to the second law of thermodynamics, the entropy of the universe increases over time as disorder increases.
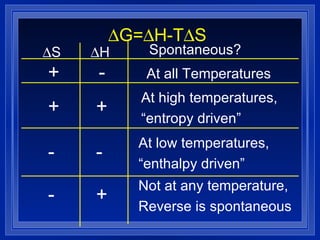
2. A spontaneous process is one whose entropy change for the universe is positive. Whether a process is spontaneous depends on the temperature and can be predicted from the sign of the enthalpy and entropy changes.
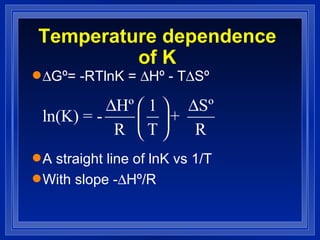
3. The free energy change of a reaction (ΔG) determines whether the reaction is spontaneous or not at a given temperature. If ΔG is negative, the reaction is spontaneous in the direction written.