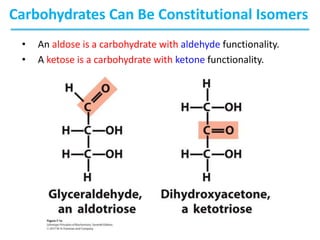
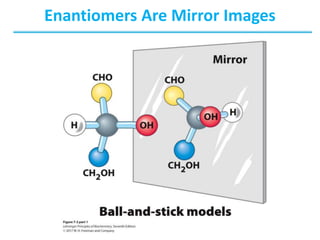
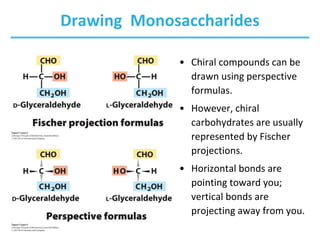
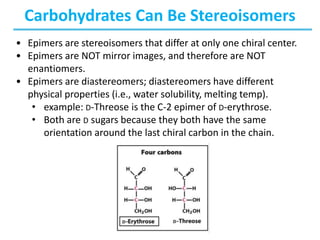
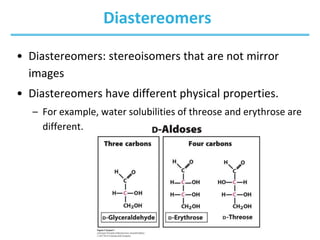
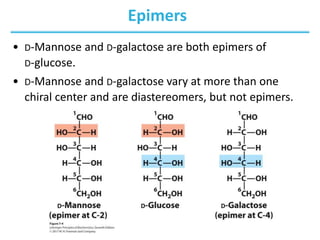
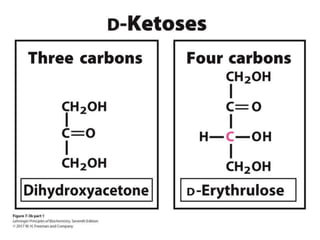
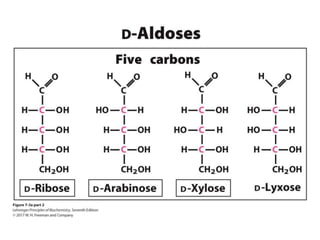
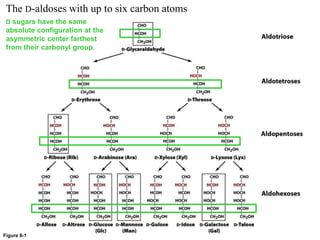
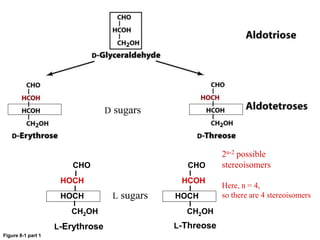
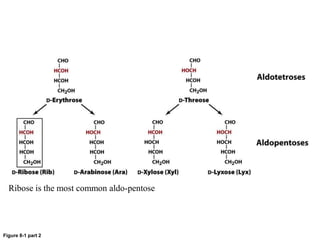
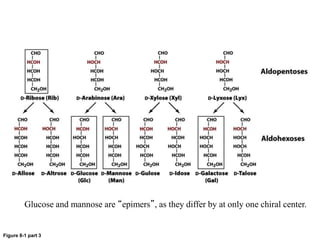
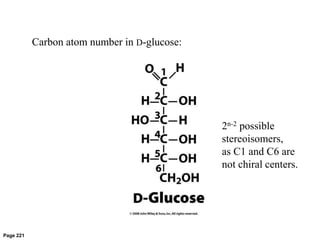
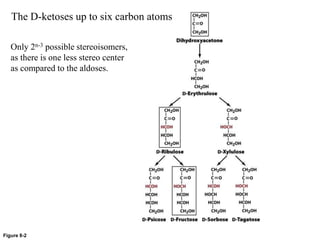
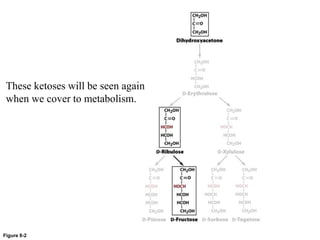
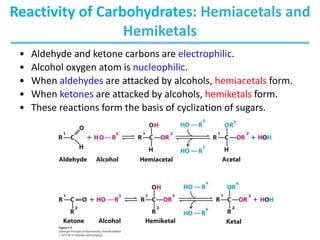
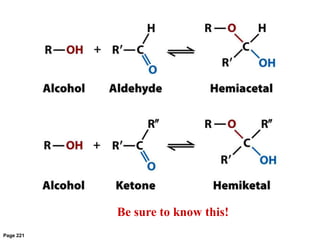
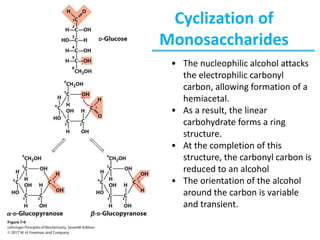
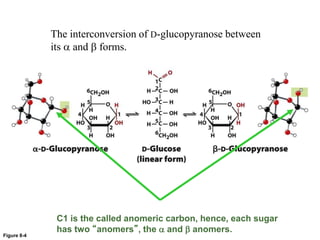
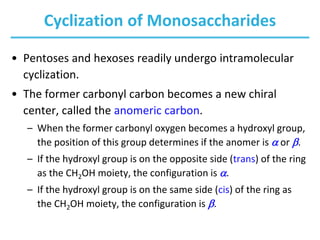
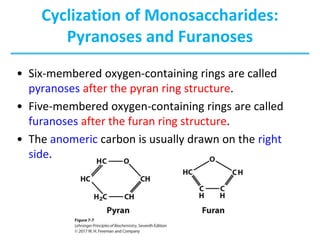
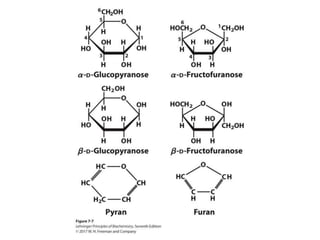
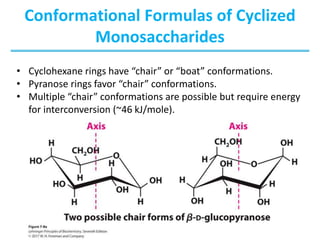
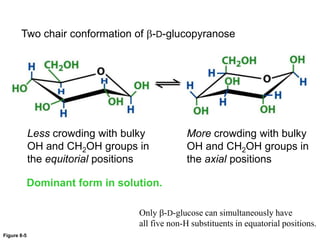
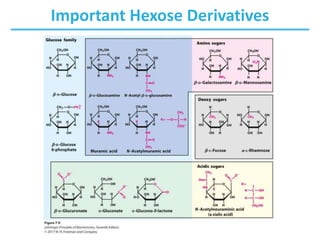
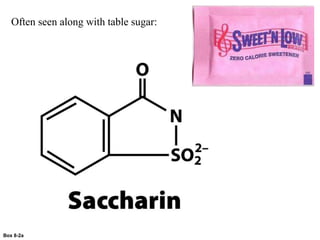
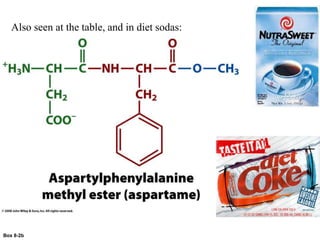
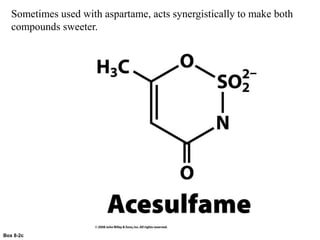
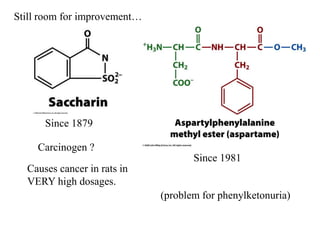
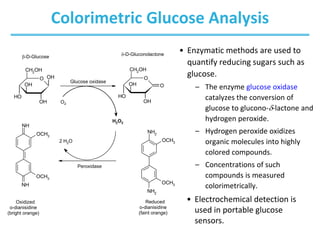
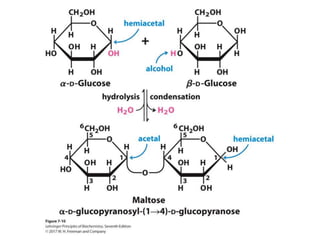
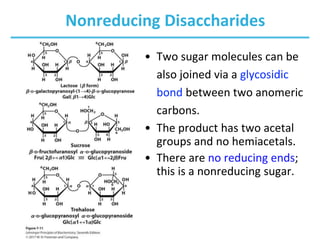
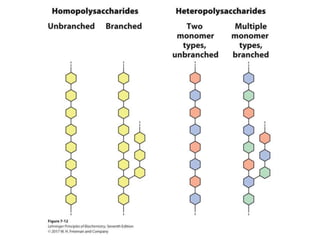
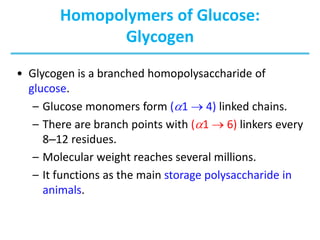
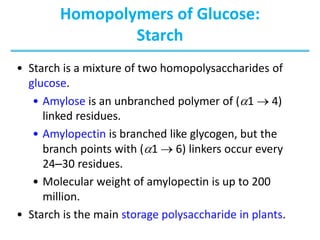
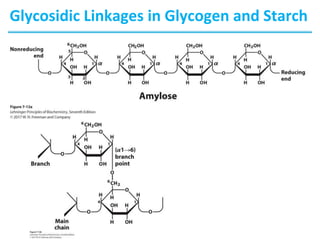
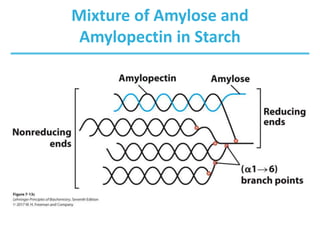
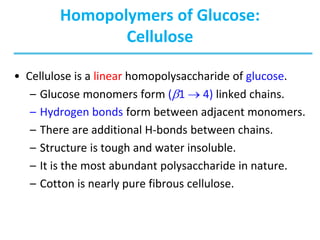
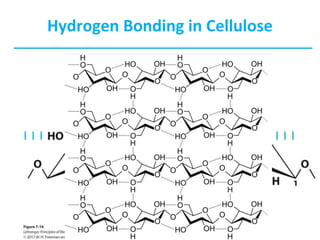
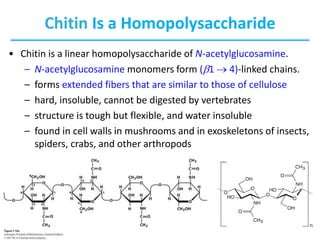
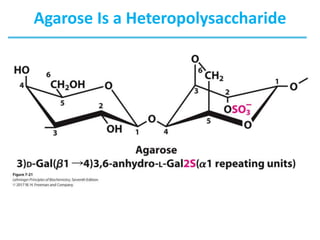
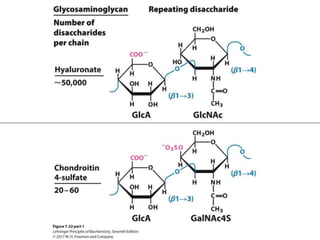
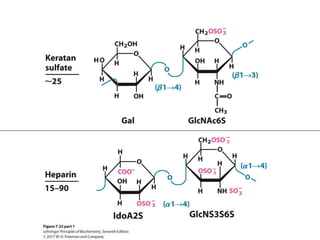
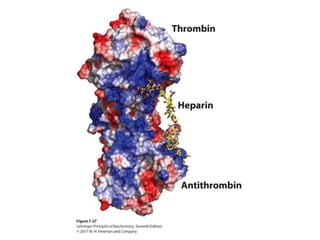
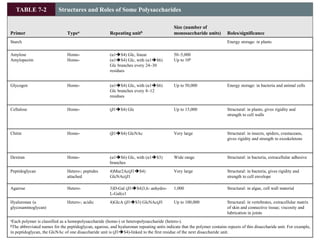
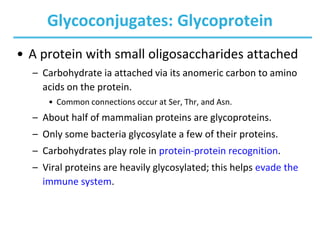
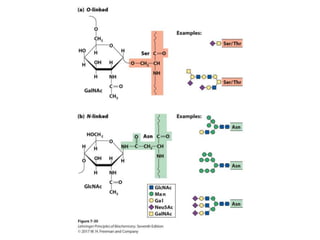
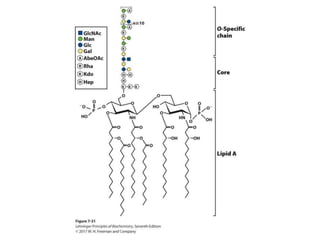
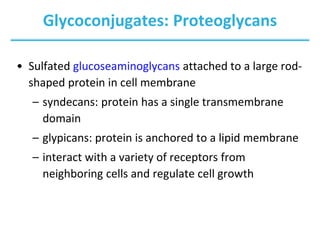
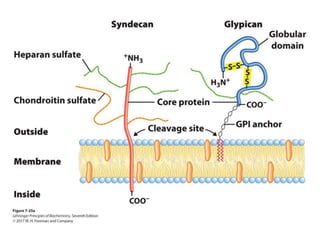
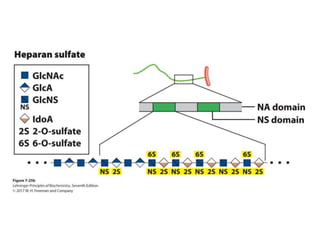
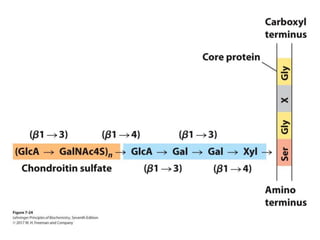
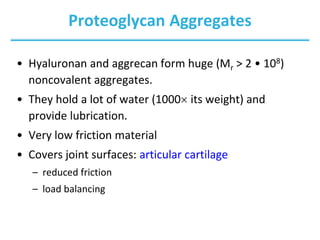
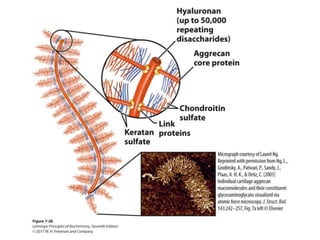
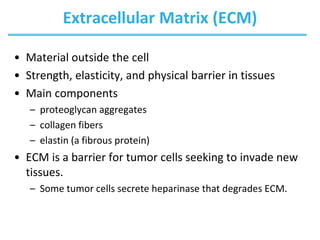
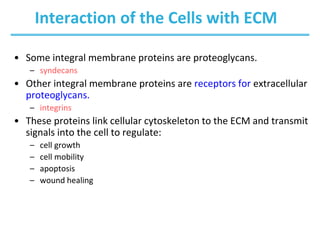
Carbohydrates are the most abundant biological molecules and serve important functions like energy storage and providing structural materials. They range in size from small molecules to very large polymers. The document discusses the structures, properties and functions of important carbohydrates like monosaccharides, disaccharides, and polysaccharides. Key points covered include the cyclization of monosaccharides to form pyranoses and furanoses, structural isomers of carbohydrates, important energy storage polysaccharides like glycogen and starch, and structural polysaccharides such as cellulose, chitin, and glycosaminoglycans.