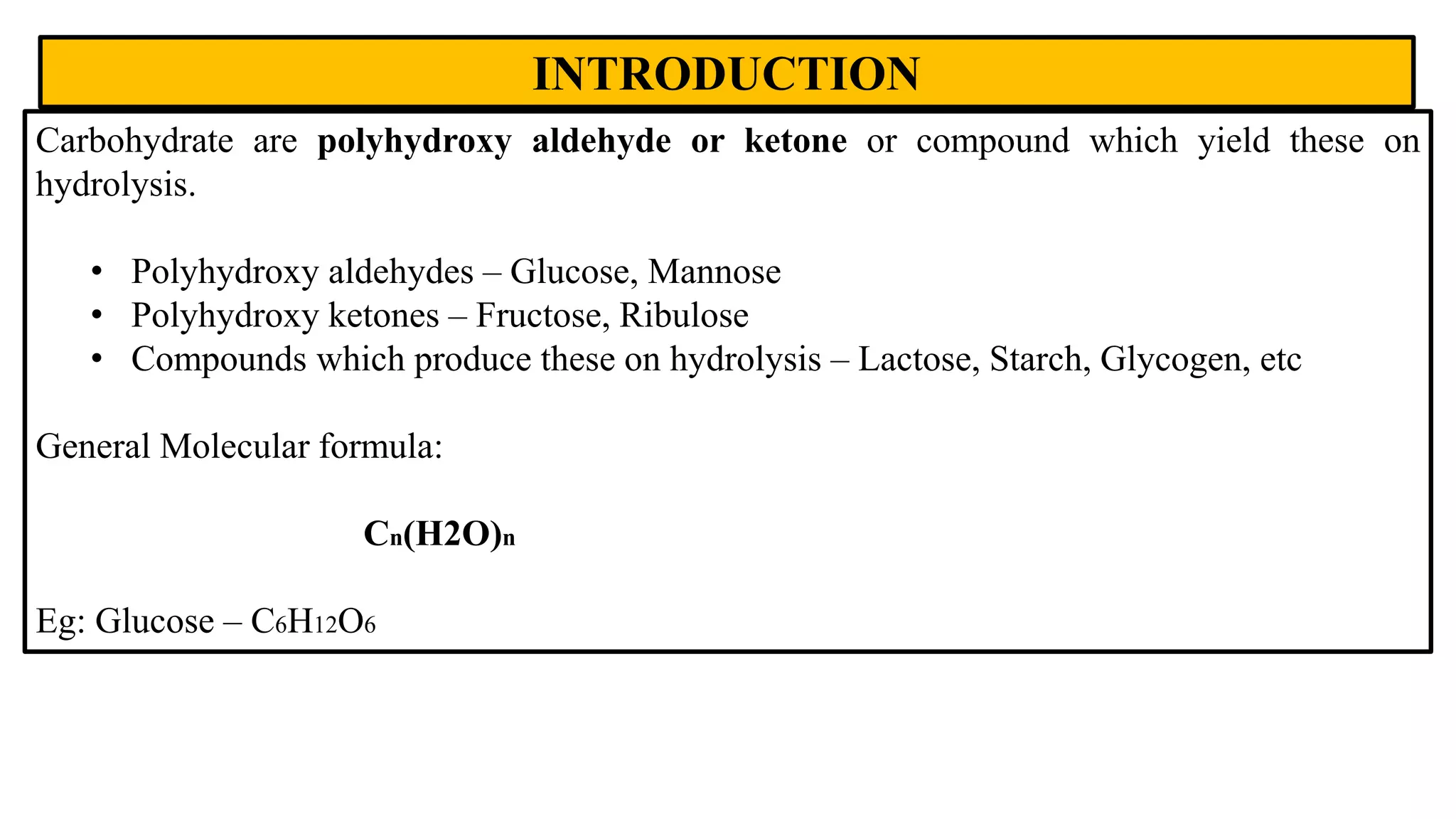
Carbohydrates are polyhydroxy aldehydes or ketones that serve important functions in the body. They provide energy, act as energy stores, and are structural components of cells. Glucose is the main energy source and is either used immediately or stored as glycogen. Other important carbohydrates include fructose, galactose, and mannose. Carbohydrates undergo various reactions and exist in multiple isomeric forms including structural isomers, stereoisomers, anomers, and epimers. Proper identification and analysis of carbohydrates is important for understanding their roles in biochemical processes.