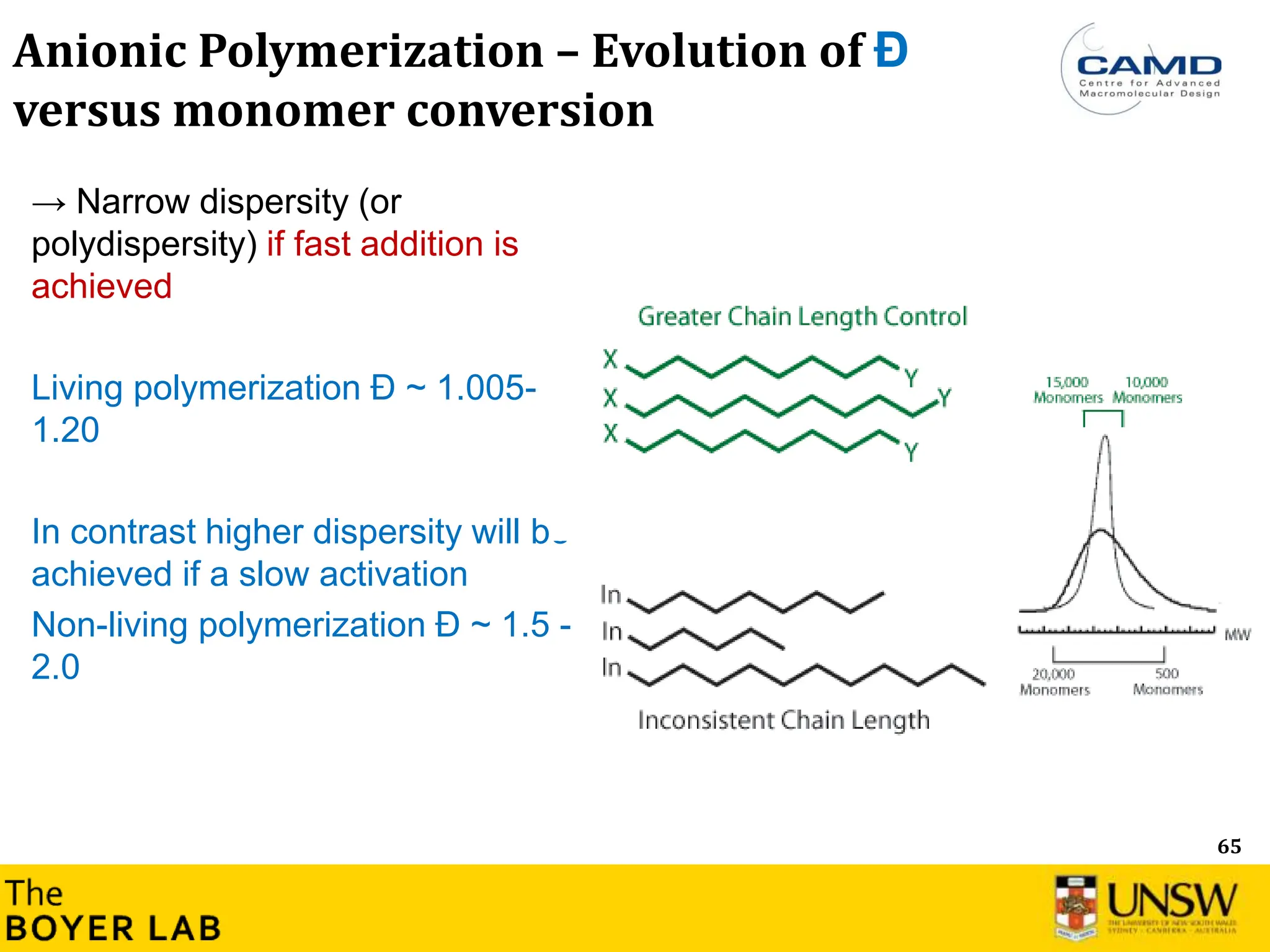
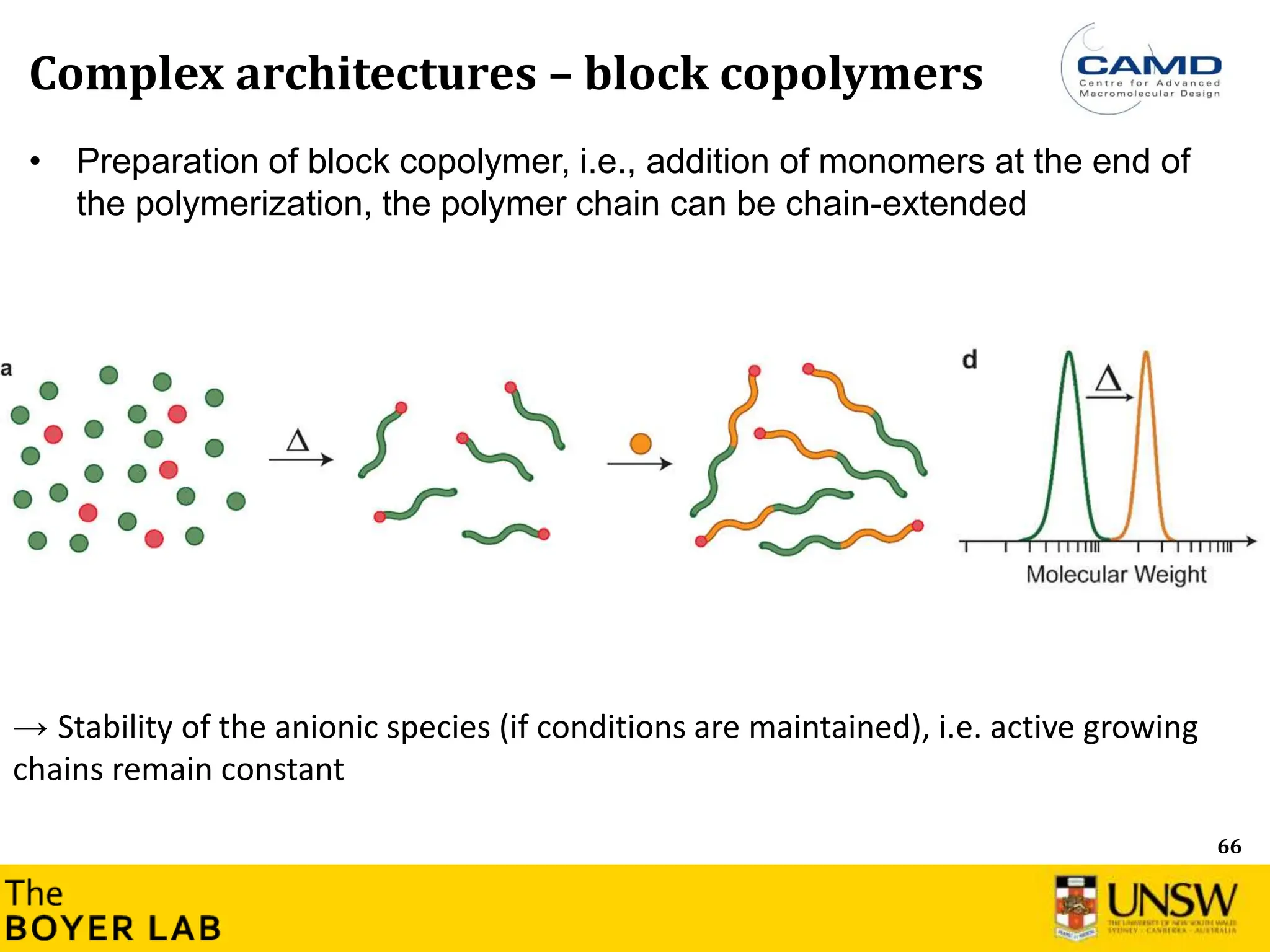
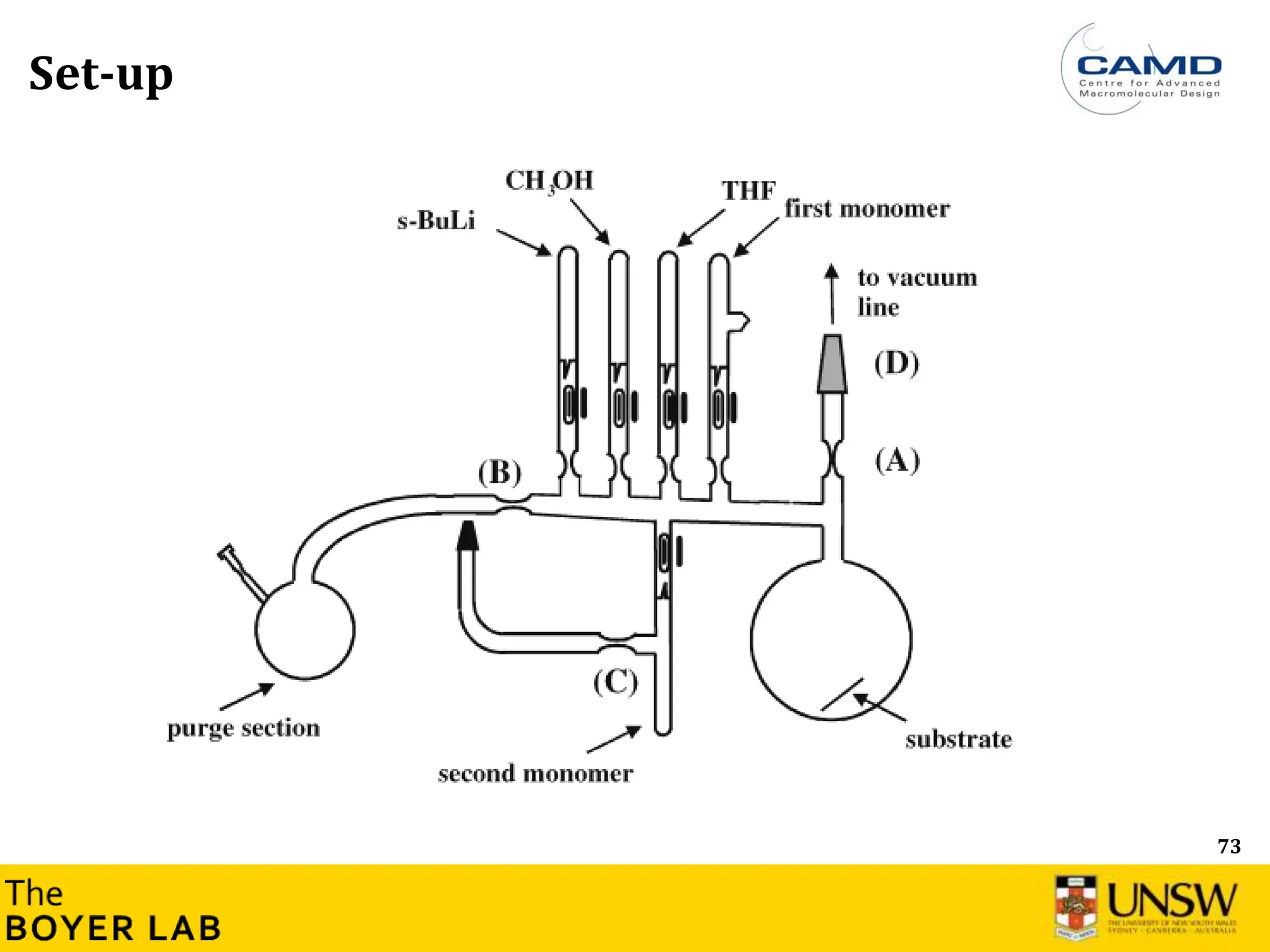
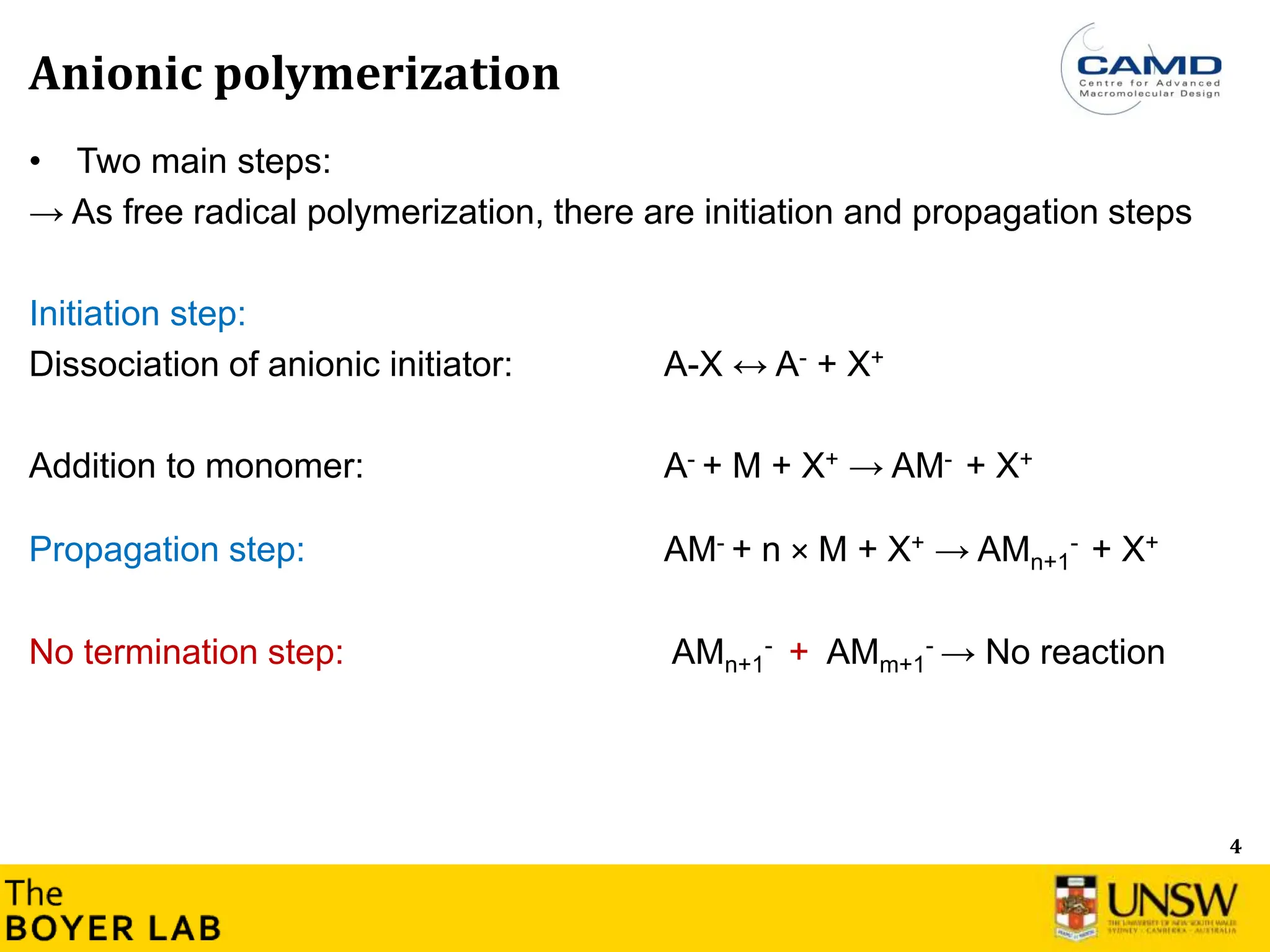
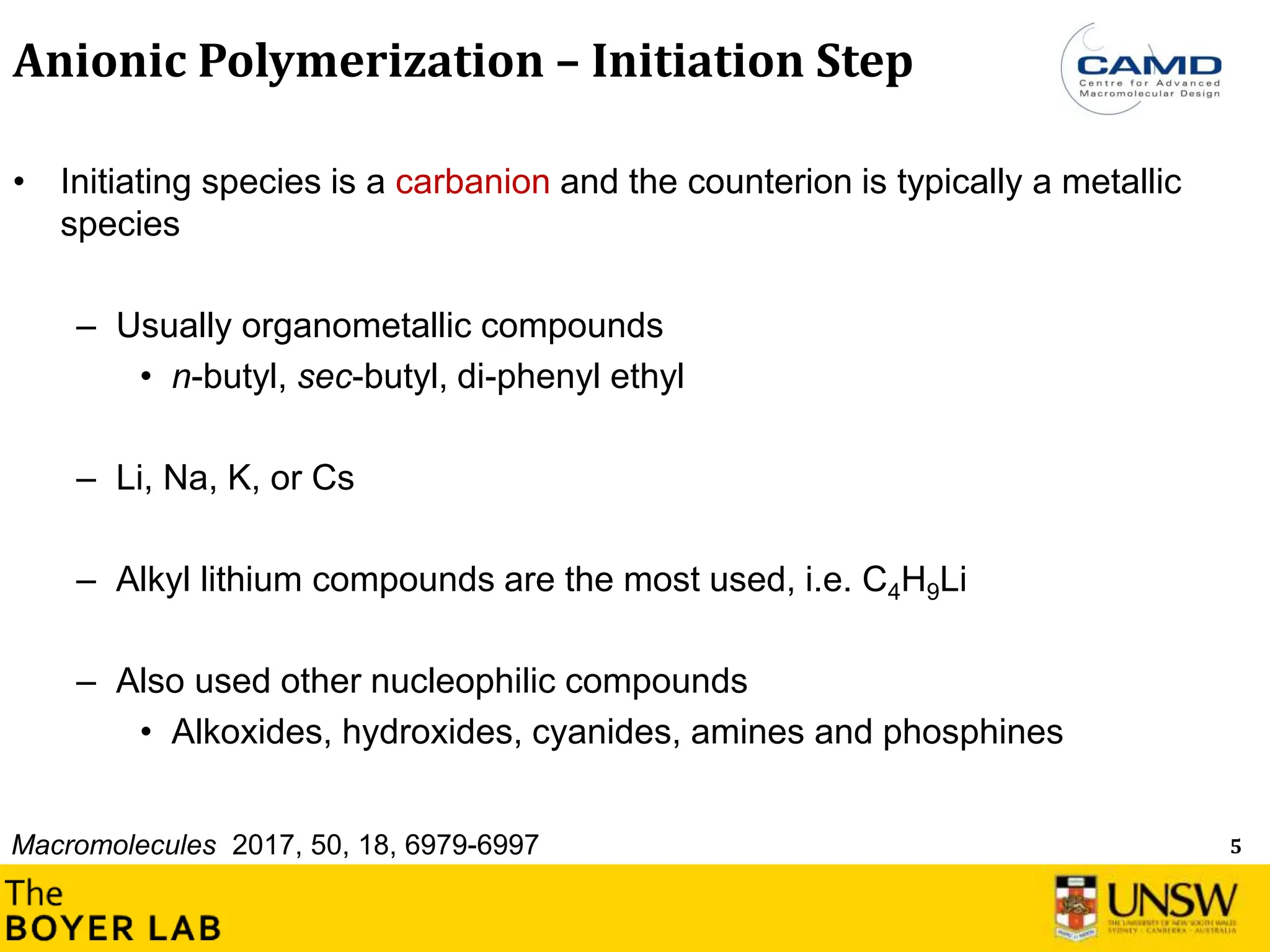
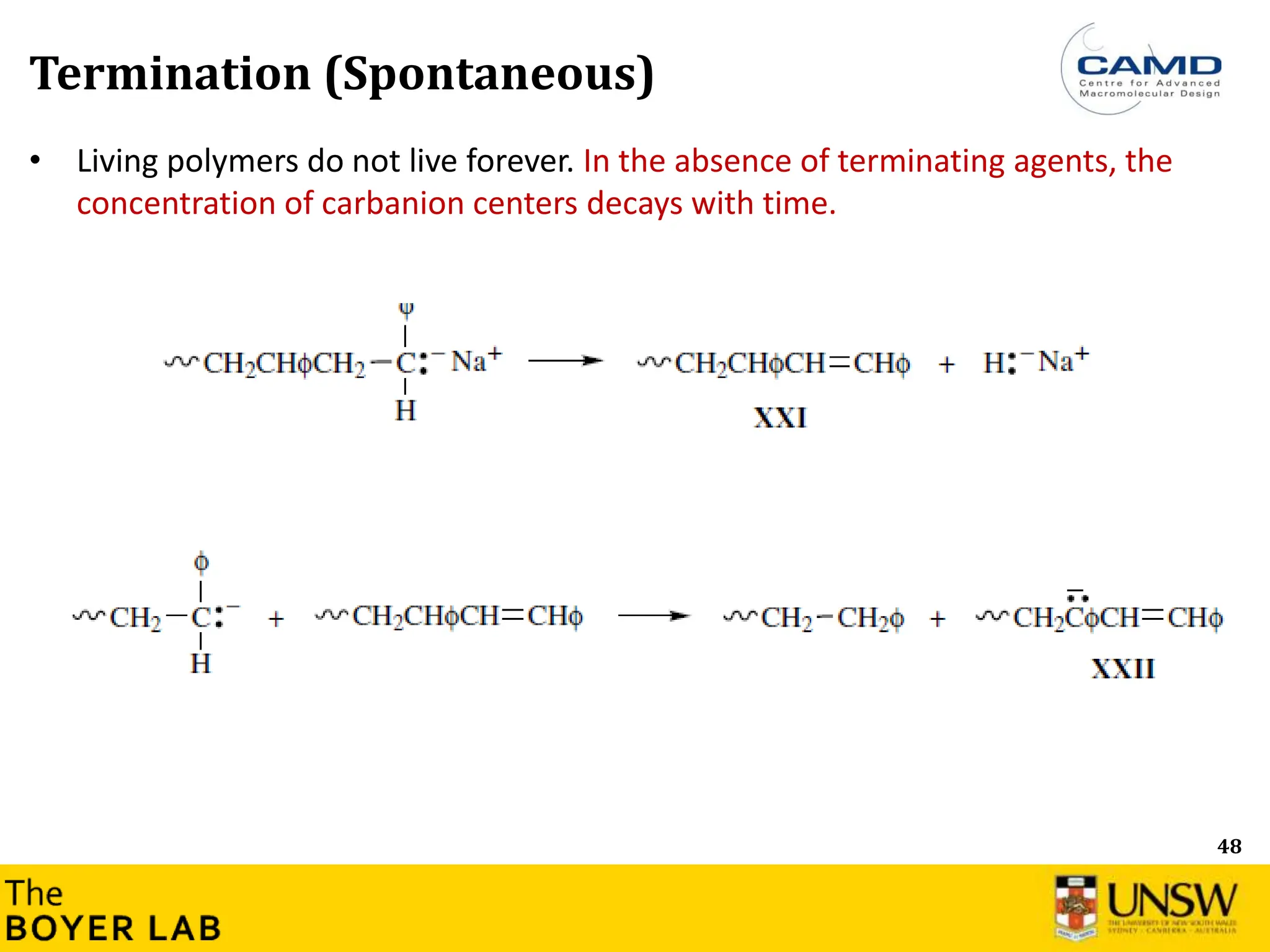
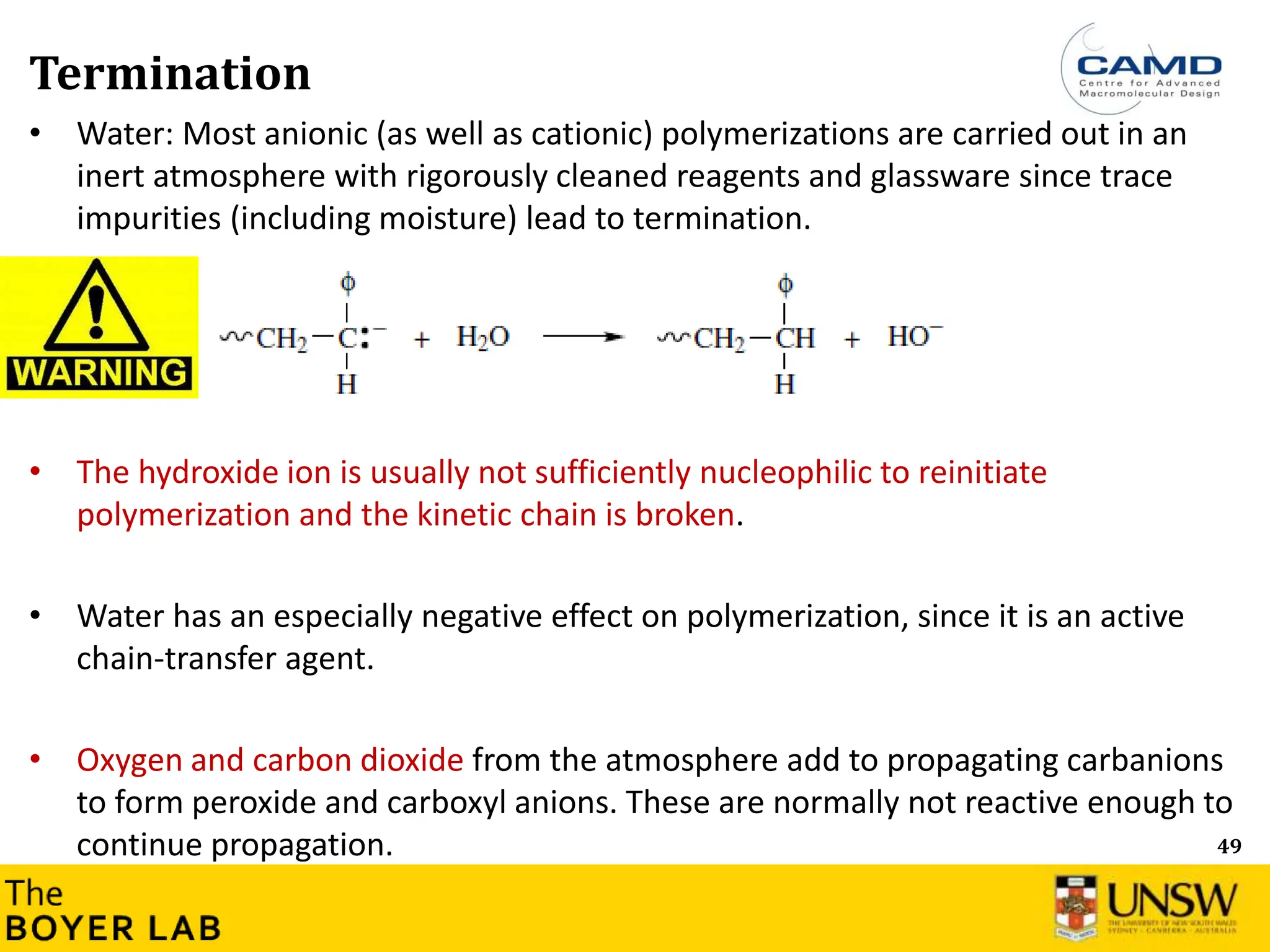
The document describes anionic polymerization, a technique developed by M. Szwarc in 1956, known for its precise molecular weight control and ability to synthesize tailored polymer architectures. Key processes include initiation using carbanion initiators and propagation without termination, allowing for the production of block and functional copolymers. Factors like initiator choice, solvent effects, and the nature of the monomers used are critical for optimizing the polymerization process and achieving desired polymer properties.


![3
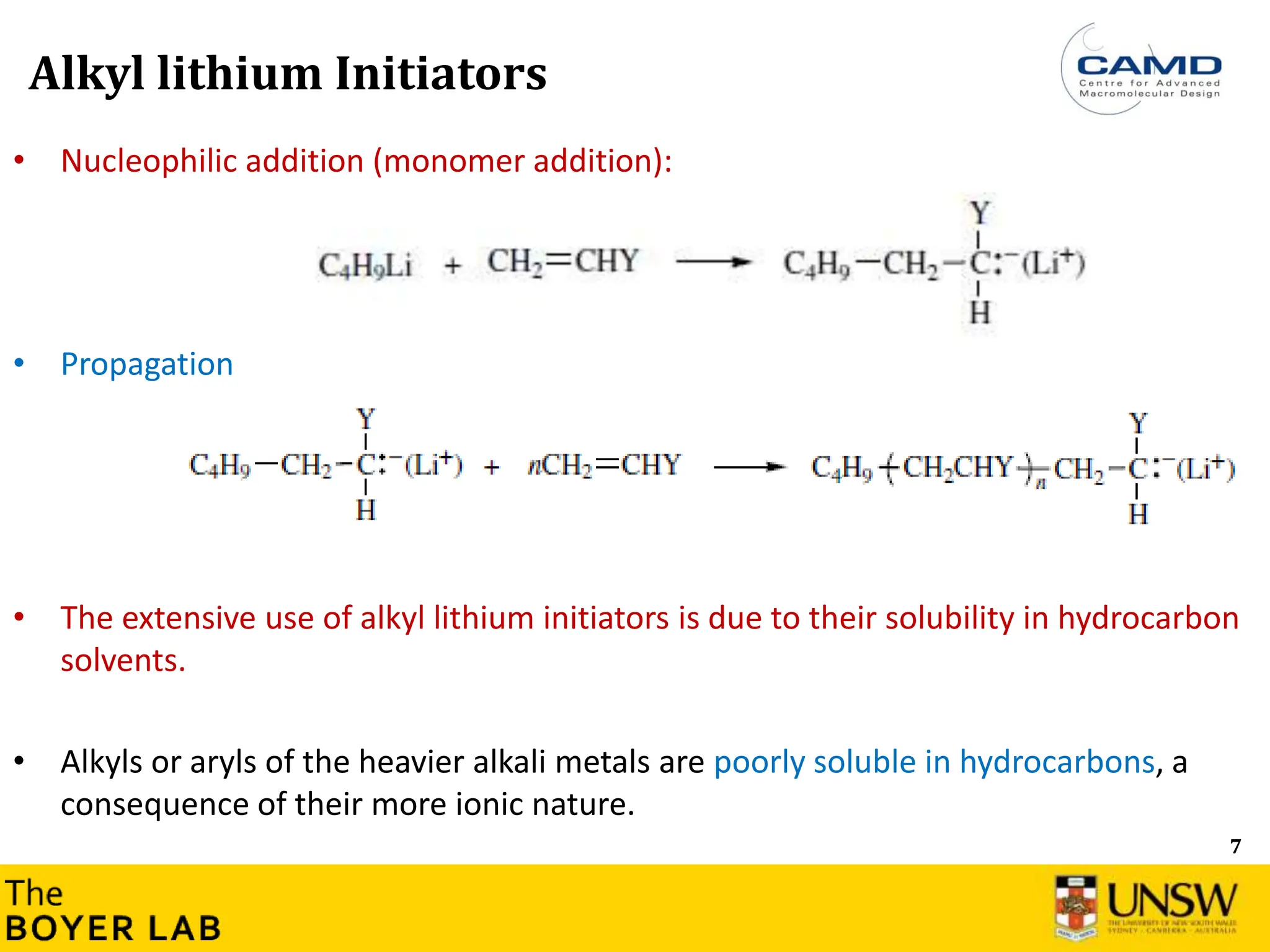
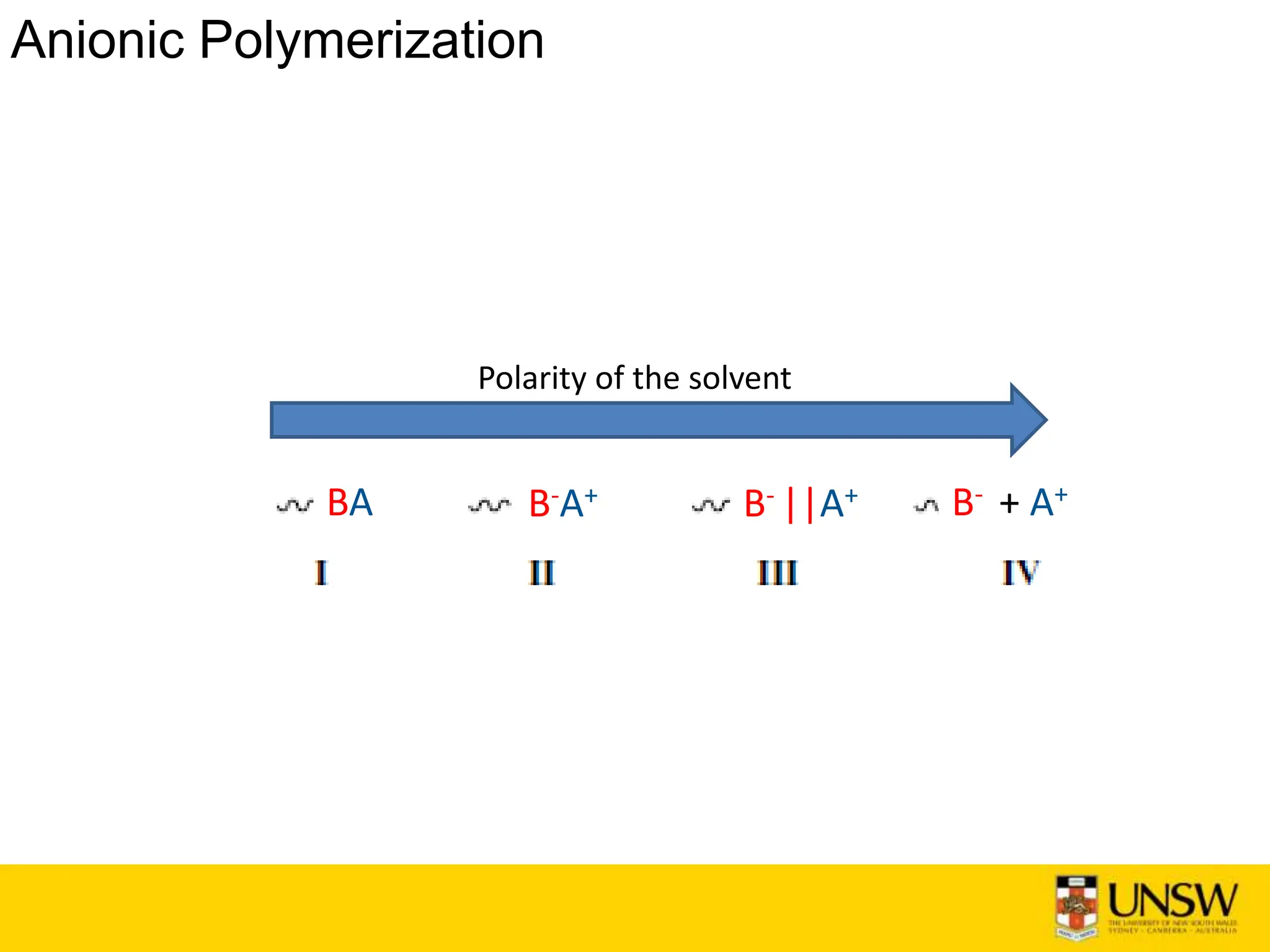
Anionic Polymerisation
• Michael Szwarc reported in the year 1956 the first anionic polymerisation
https://onlinelibrary.wiley.com/doi/full/10.1002/macp.201700217
Lowest dispersity of all synthetic methods
known, described by a Poisson distribution.
Excellent molecular weight control by the
ratio of monomer and initiator, [M]/[I]
High and even extremely high molecular
weights exceeding 106 g mol–1 can be
achieved.
Complete chain end functionalization is
possible, enabling the synthesis of
AB‐diblock, ABC‐triblock and even
(AB)n‐multiblock copolymers as well as a
broad range of precisely end‐functionalized
polymers. M. Szwarc, Nature 1956, 178, 1168.](https://image.slidesharecdn.com/anionicpolymerization2024part2-240626111321-d24bca81/75/Anionic-Polymerization-2024-Chemistry-course-3-2048.jpg)
![8
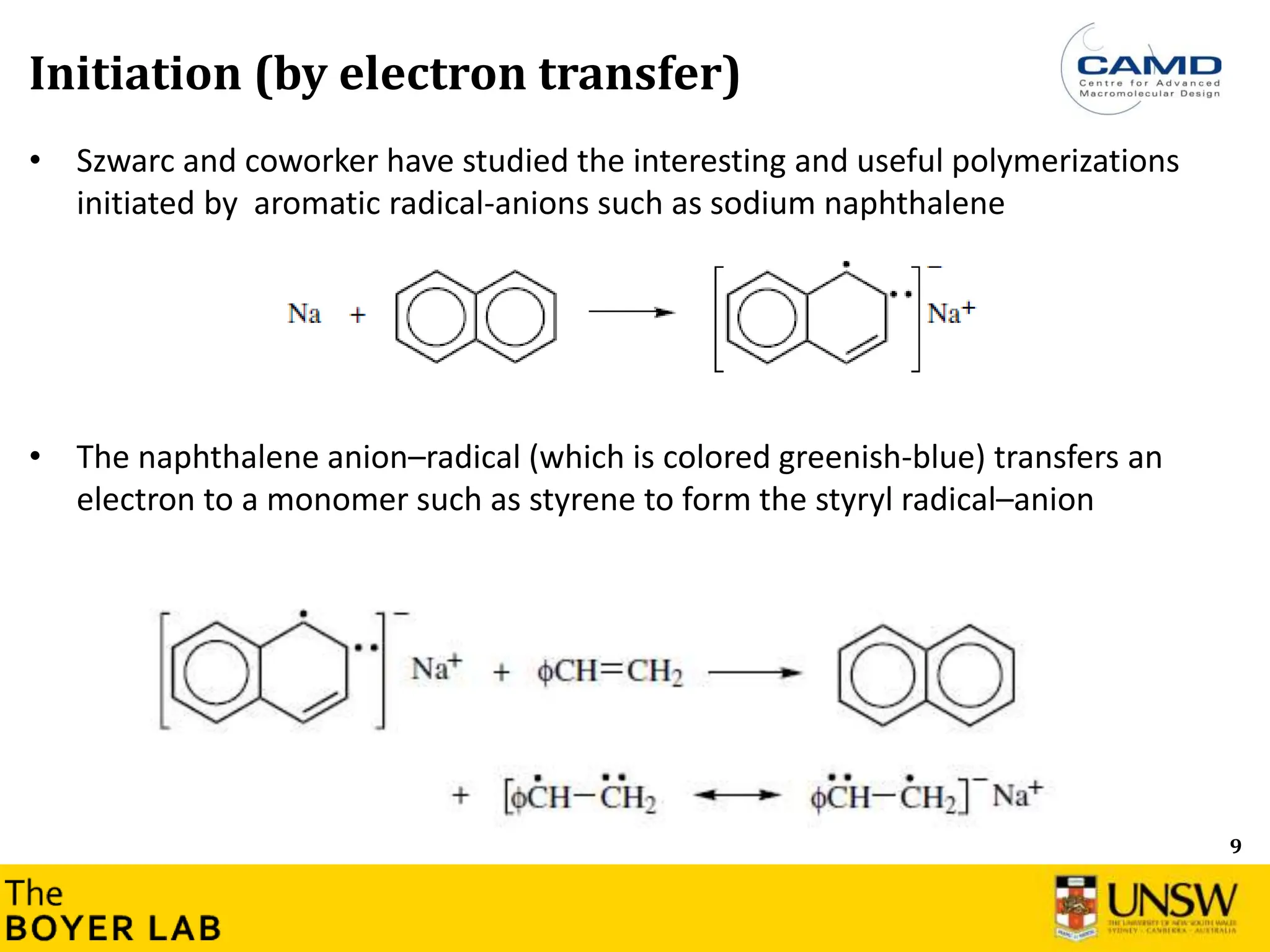
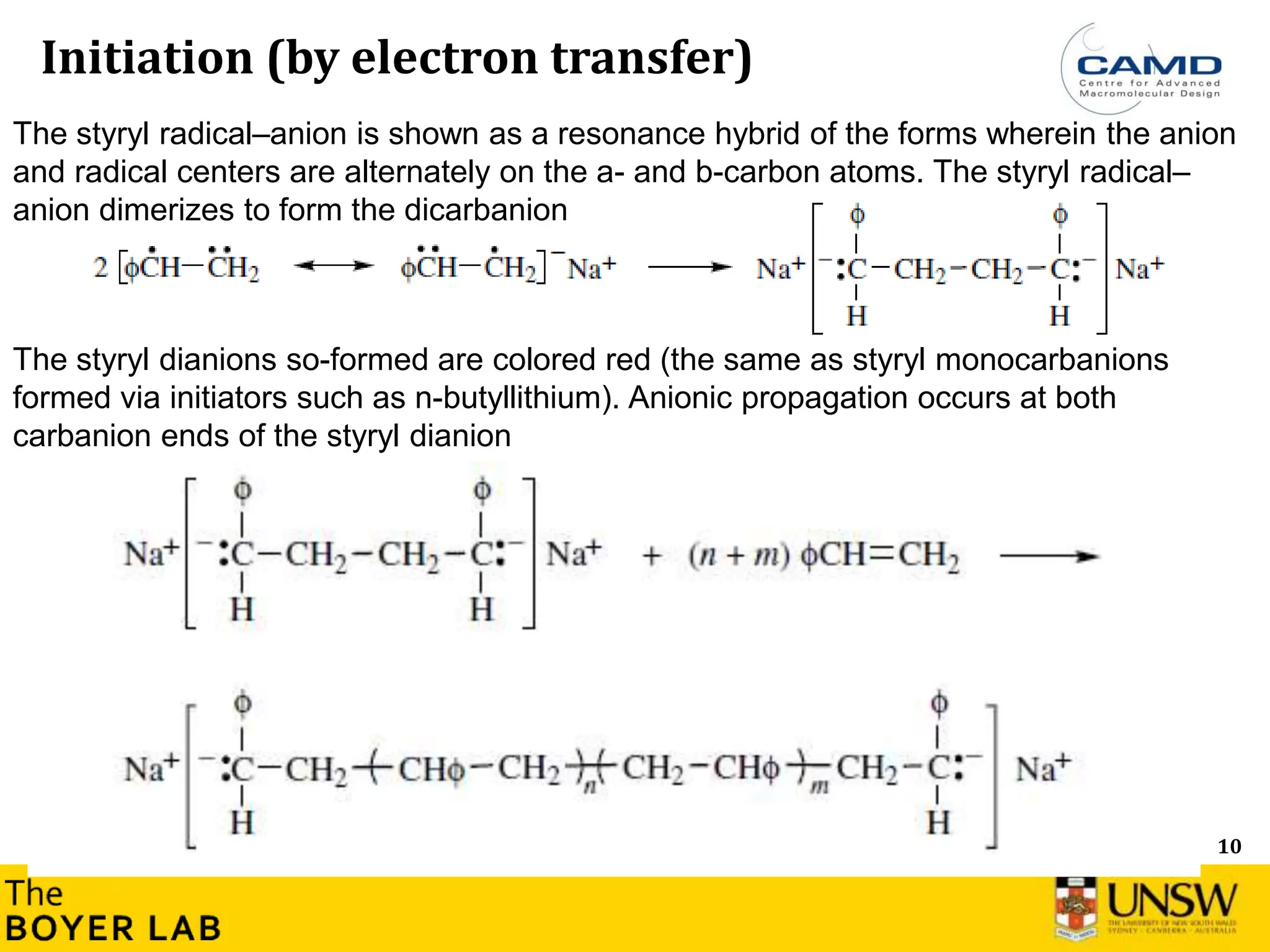
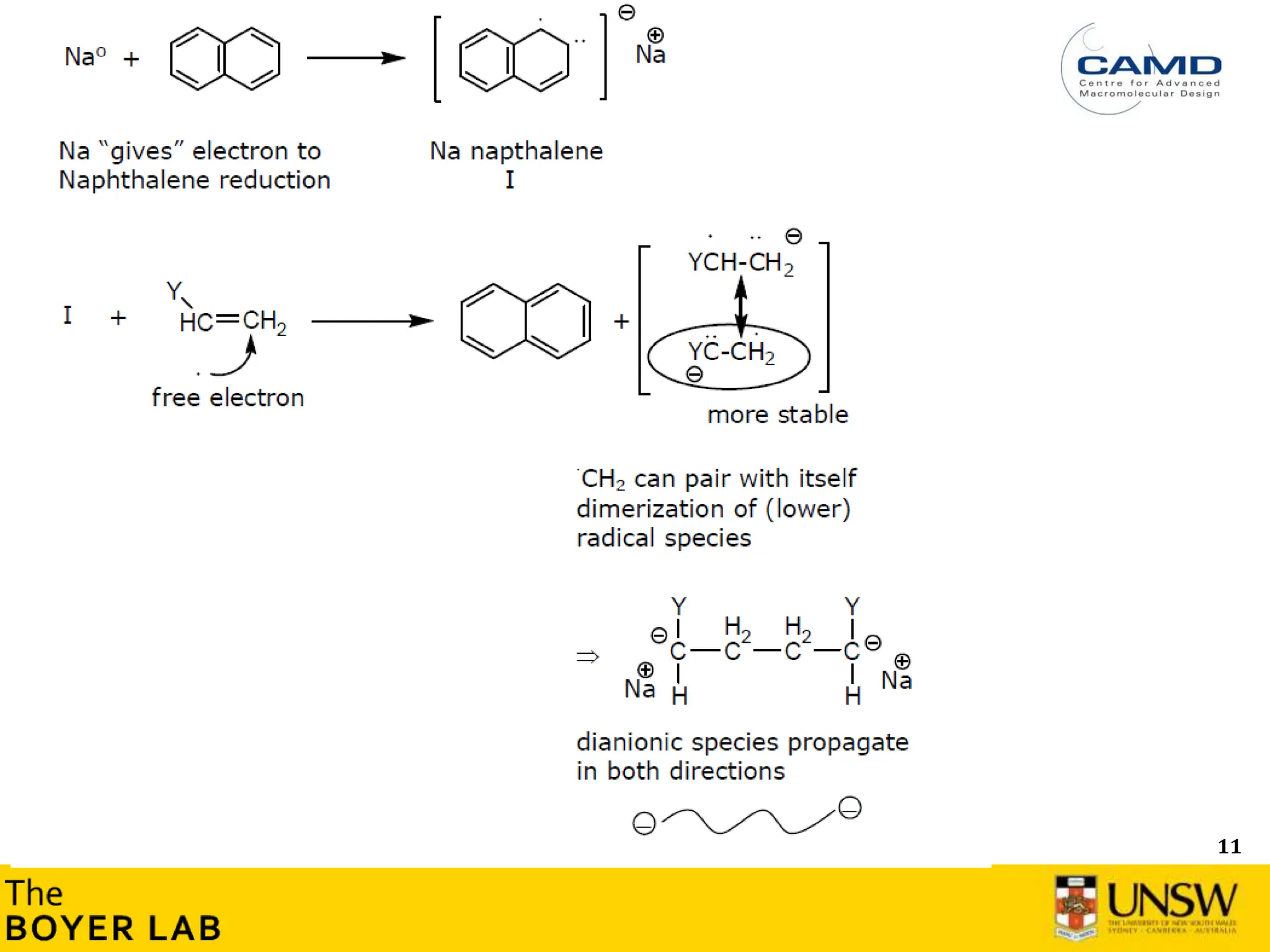
Initiation and propagation
What is the consequence on the consumption of the initiator during the
polymerisation? Can you plot [initiator] versus time (qualitatively)?
How this compares with radical polymerisation? Can you plot [Initiator]
versus time (qualitatively)?
→ Initiation step is usually very fast and much faster than the propagation in anionic
polymerisation](https://image.slidesharecdn.com/anionicpolymerization2024part2-240626111321-d24bca81/75/Anionic-Polymerization-2024-Chemistry-course-8-2048.jpg)

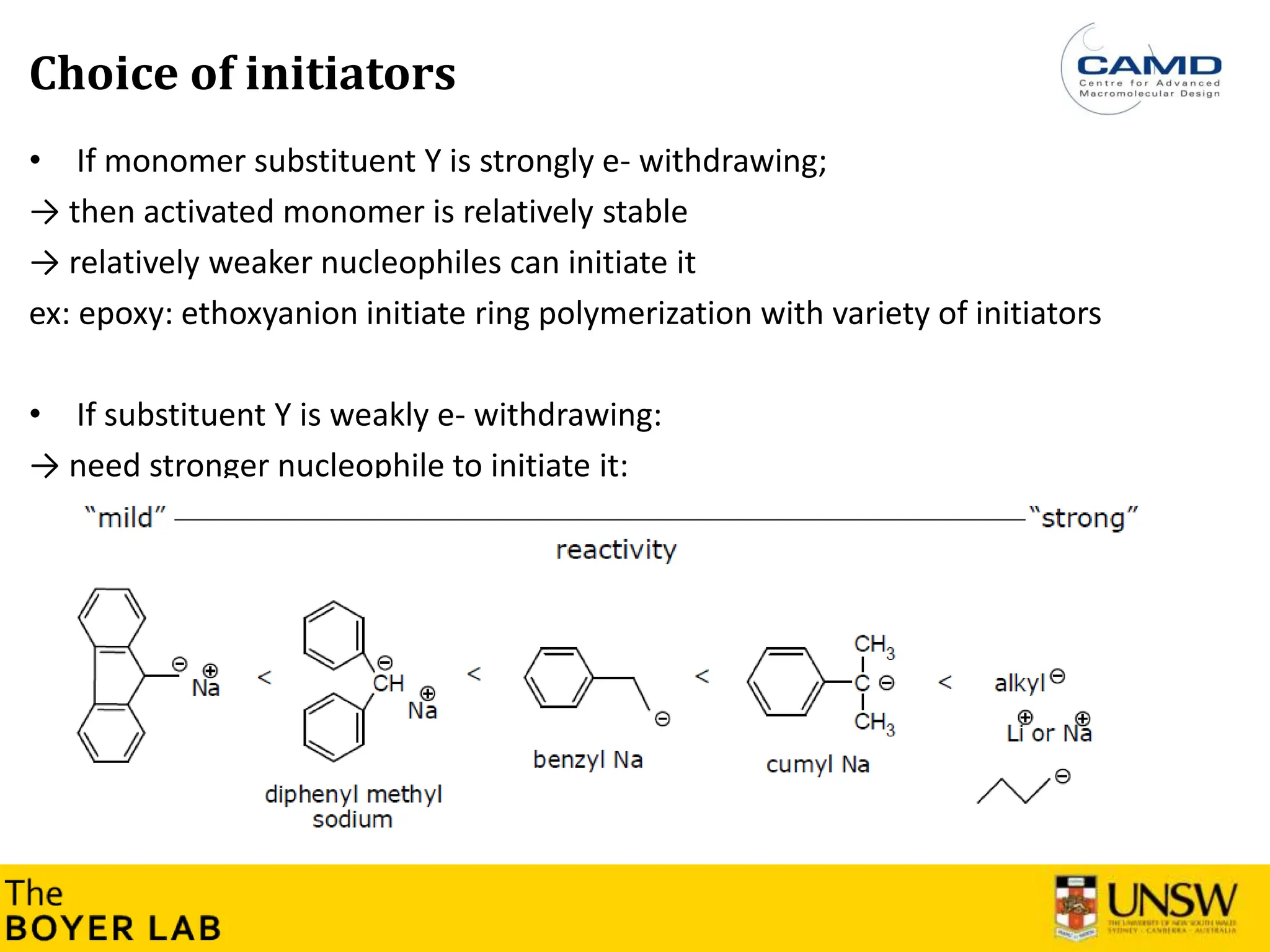

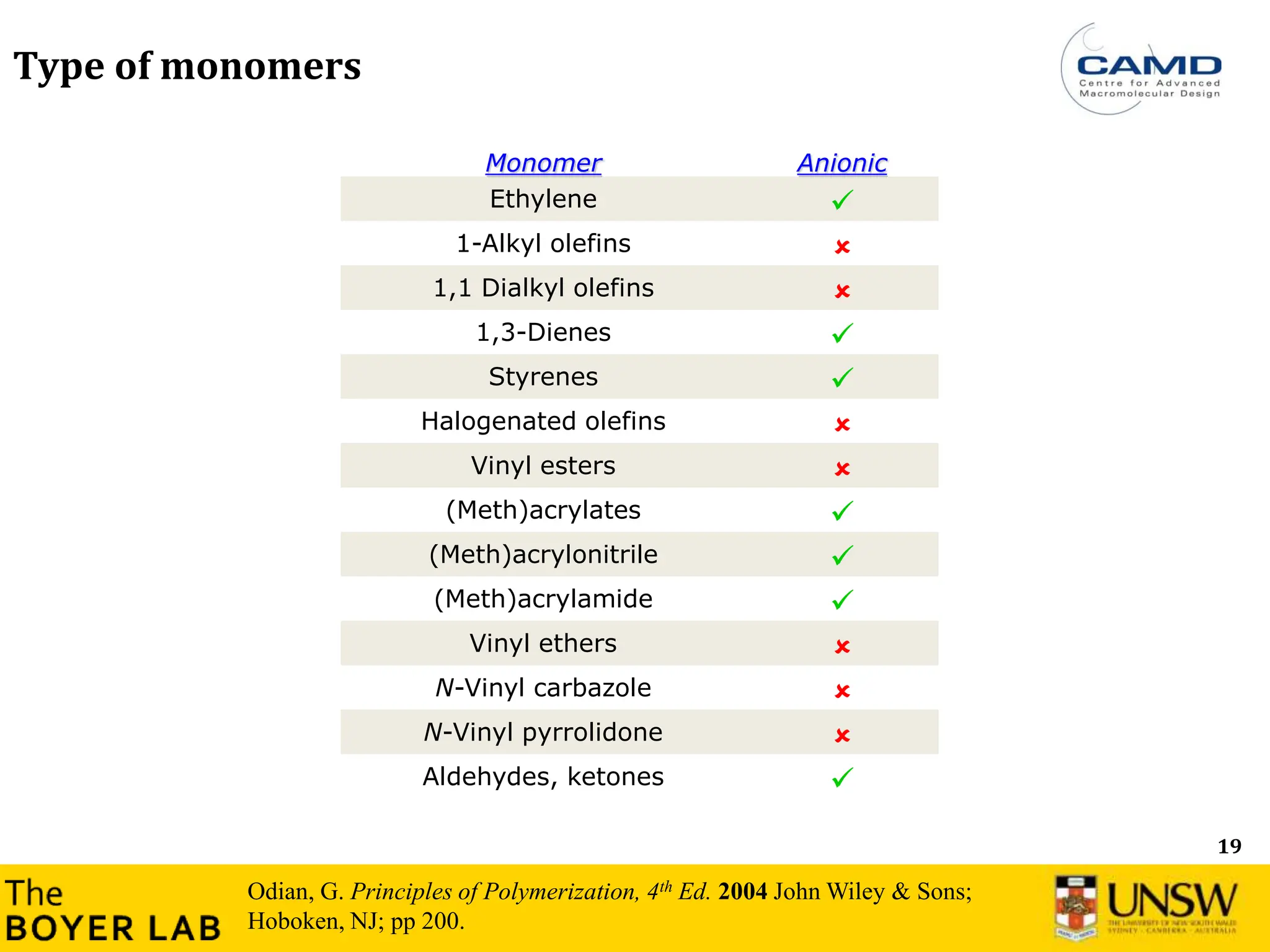
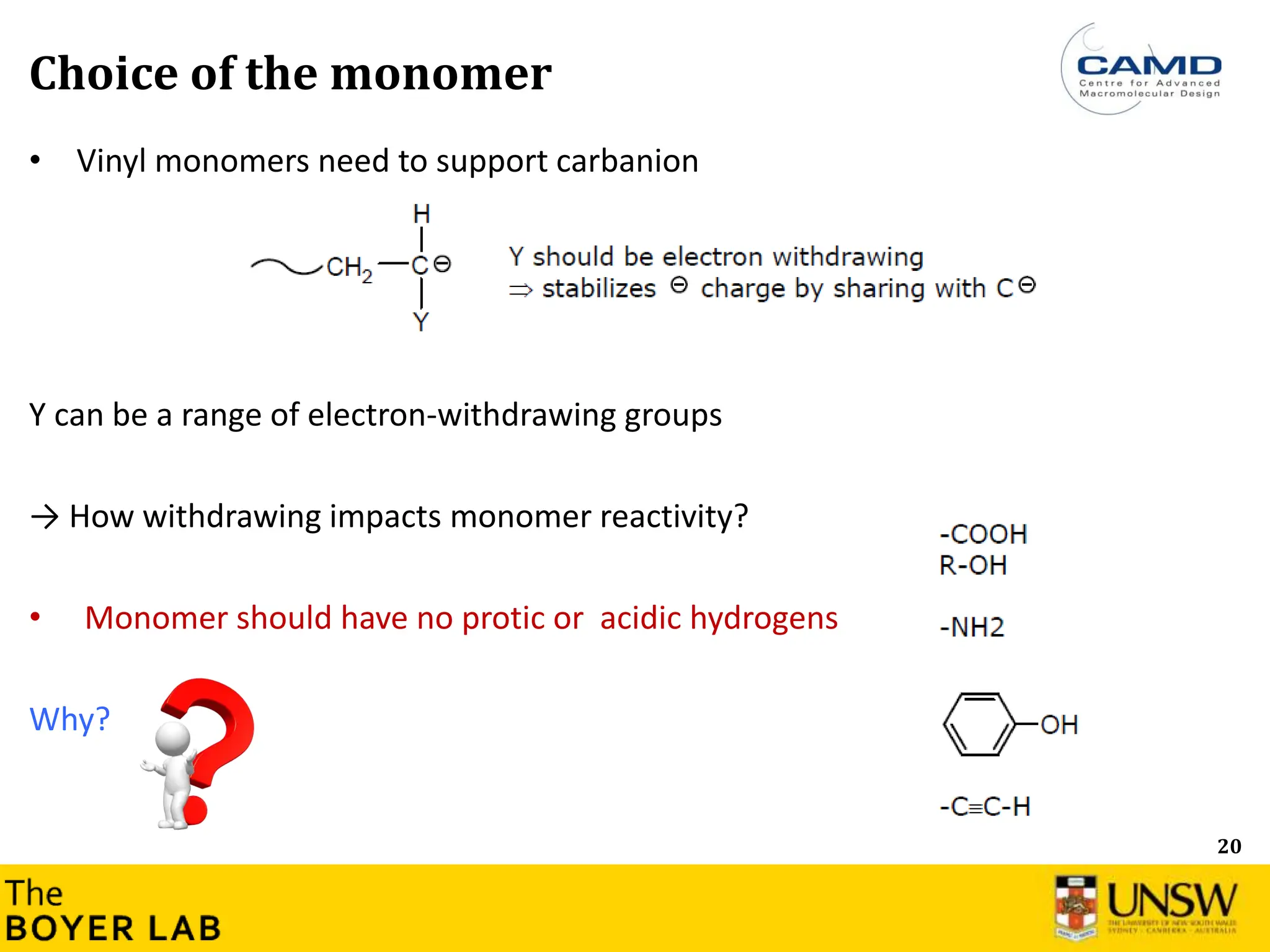
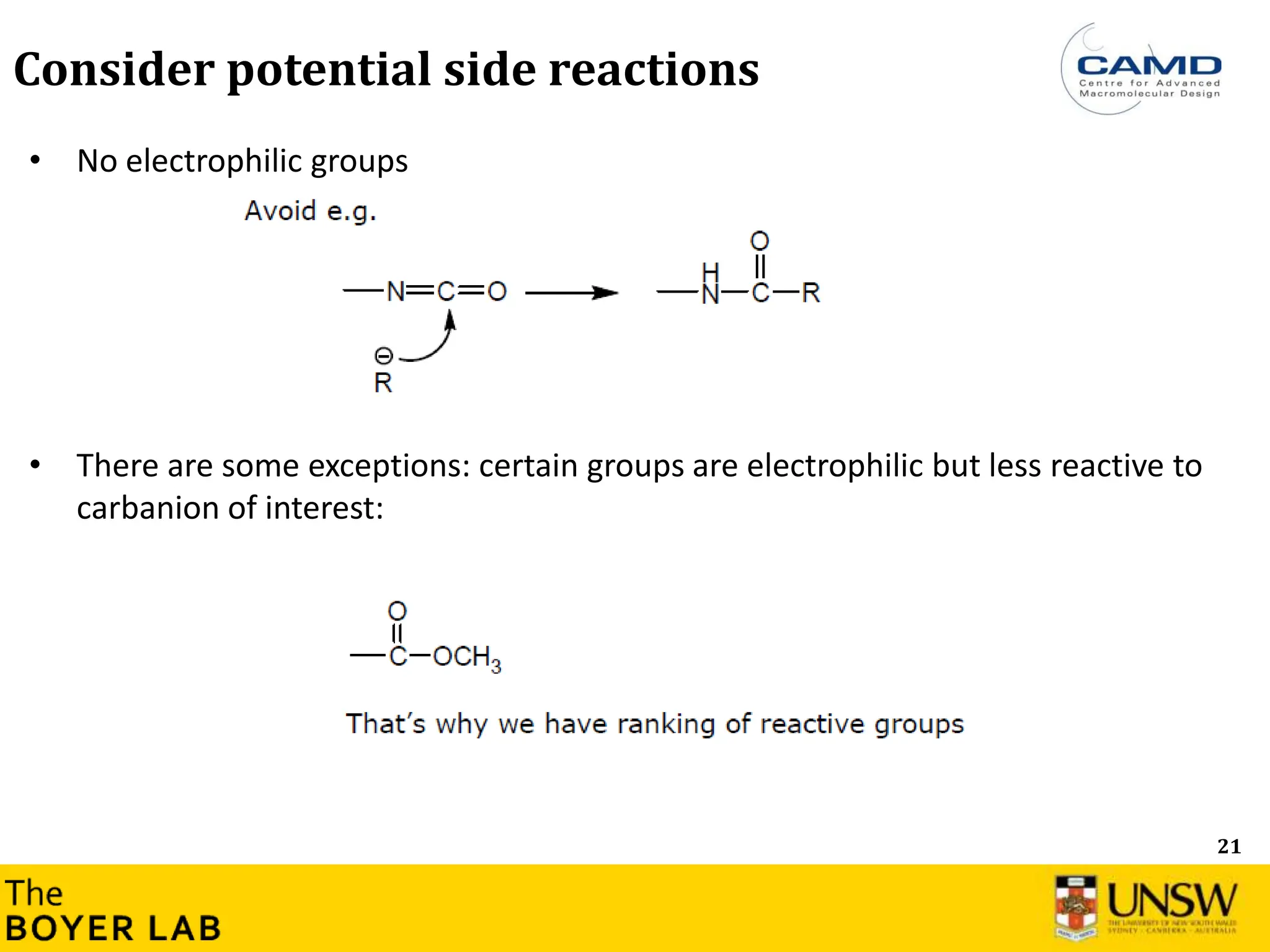
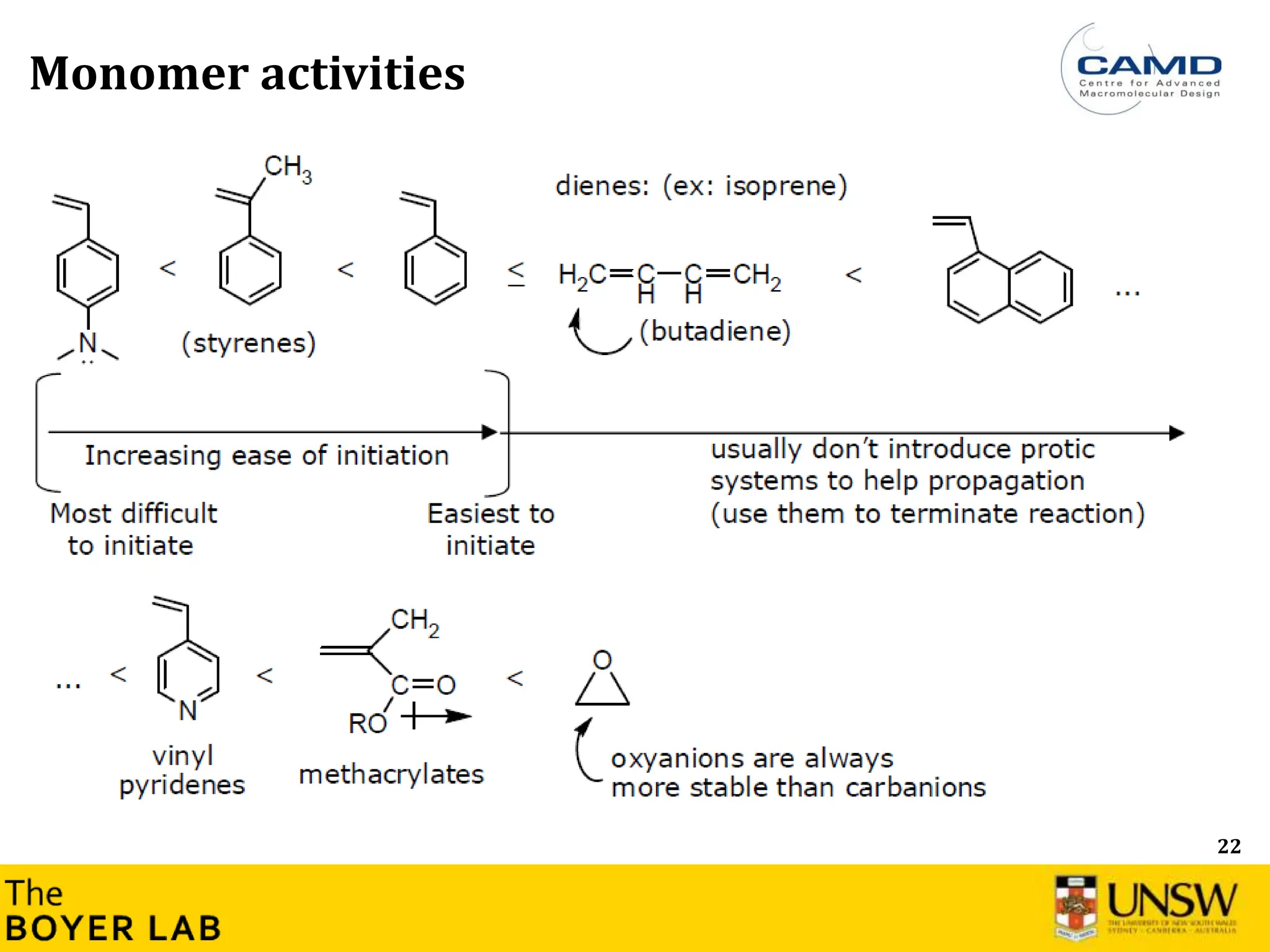
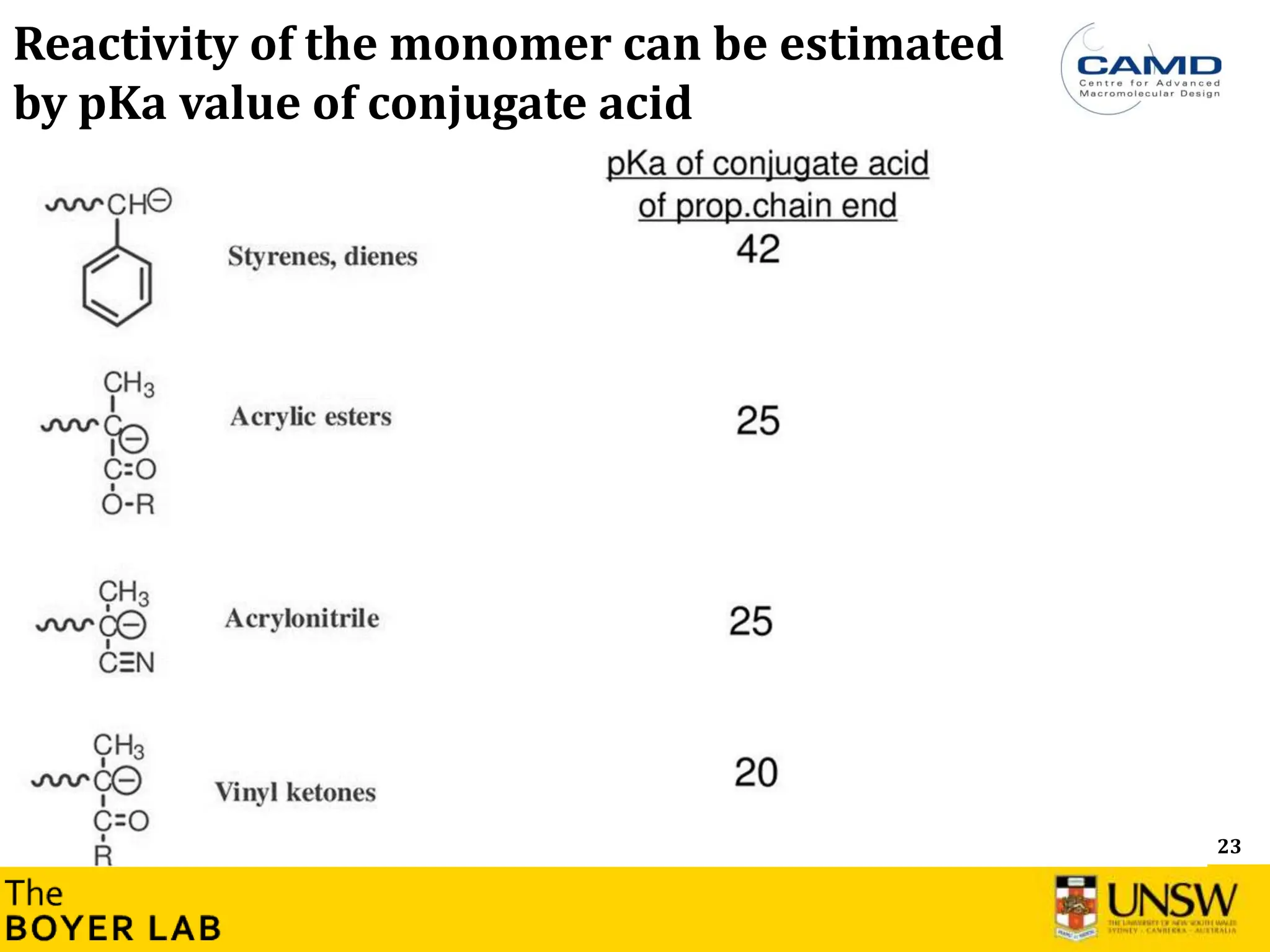
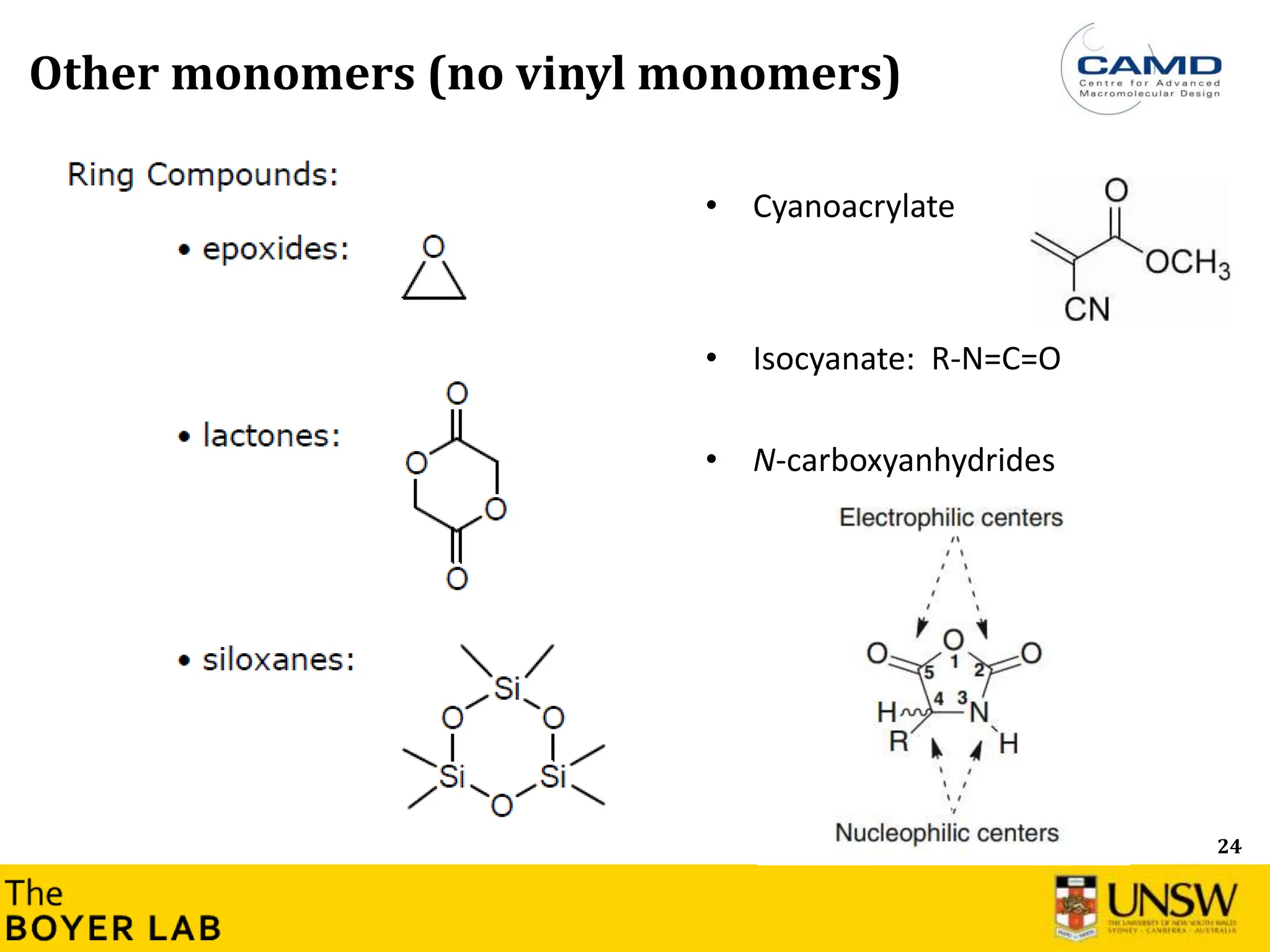
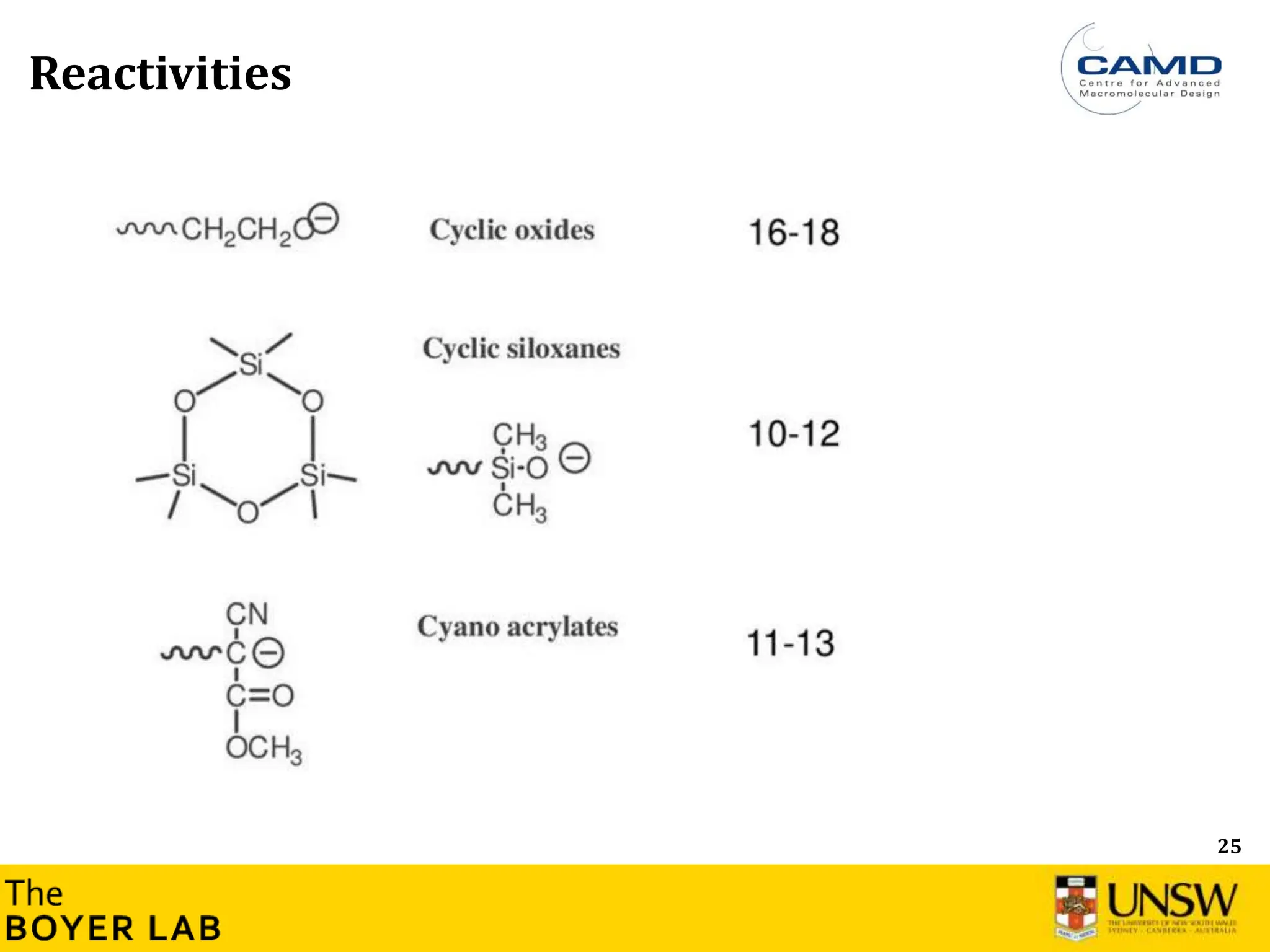
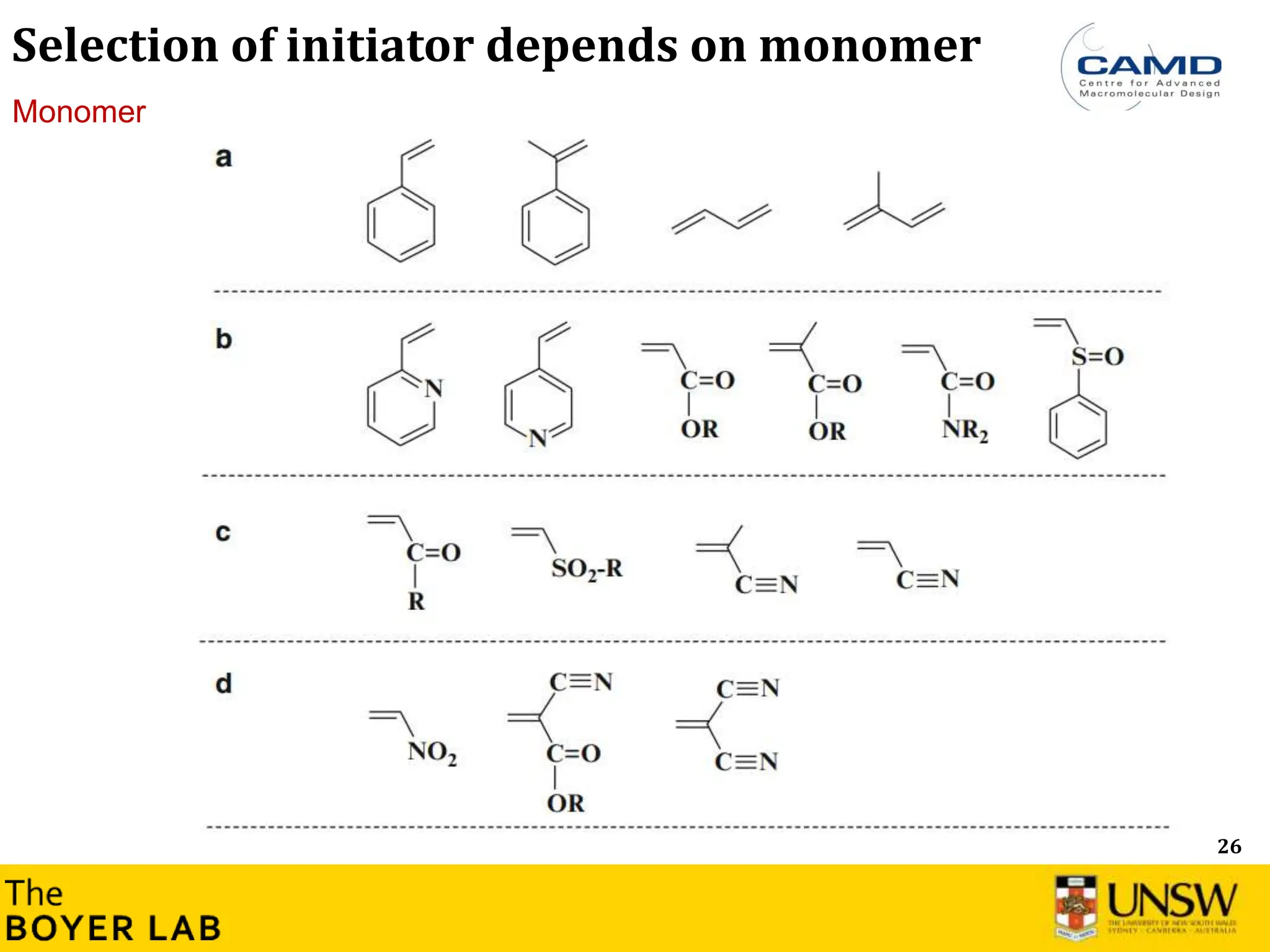
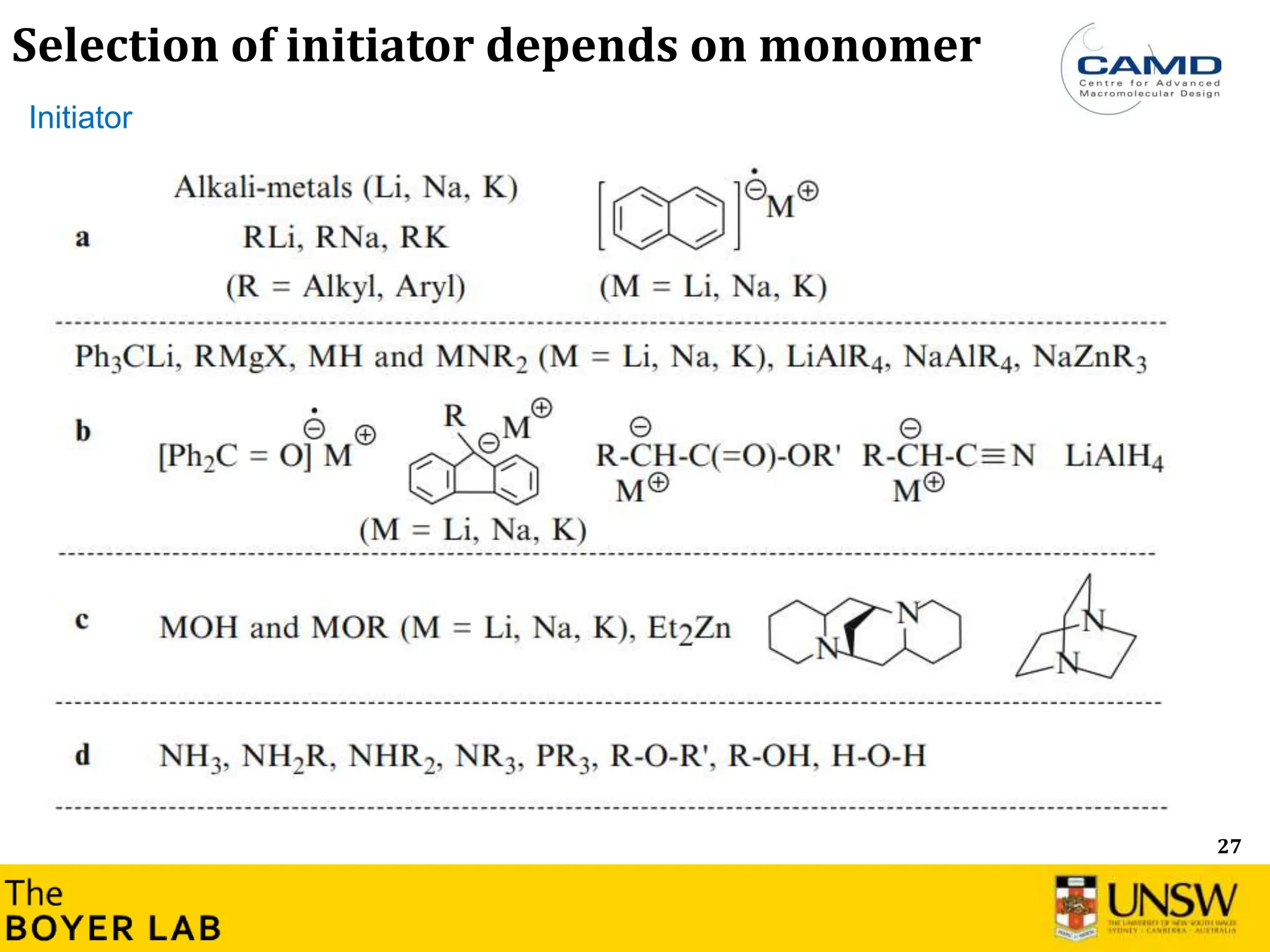
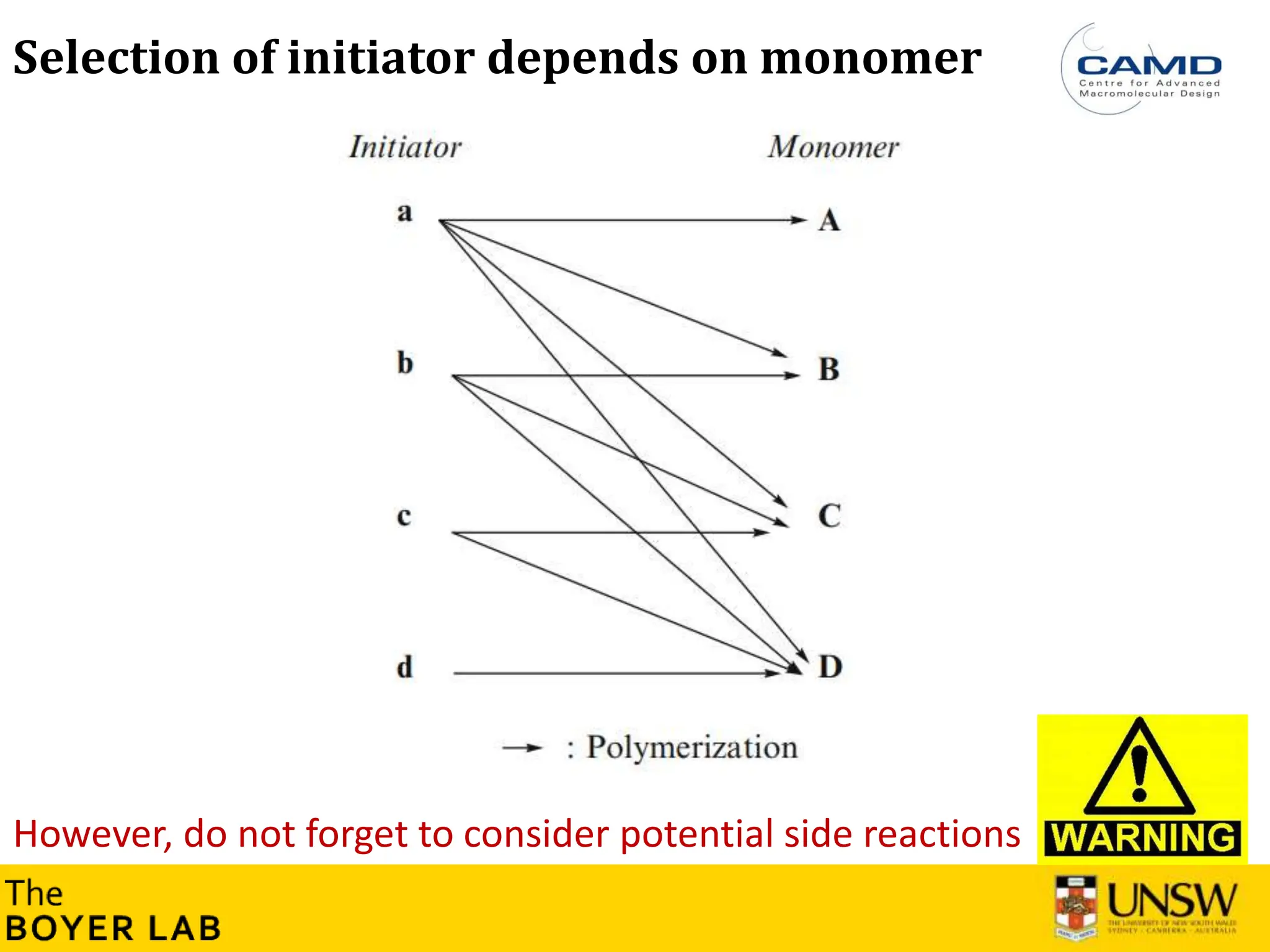
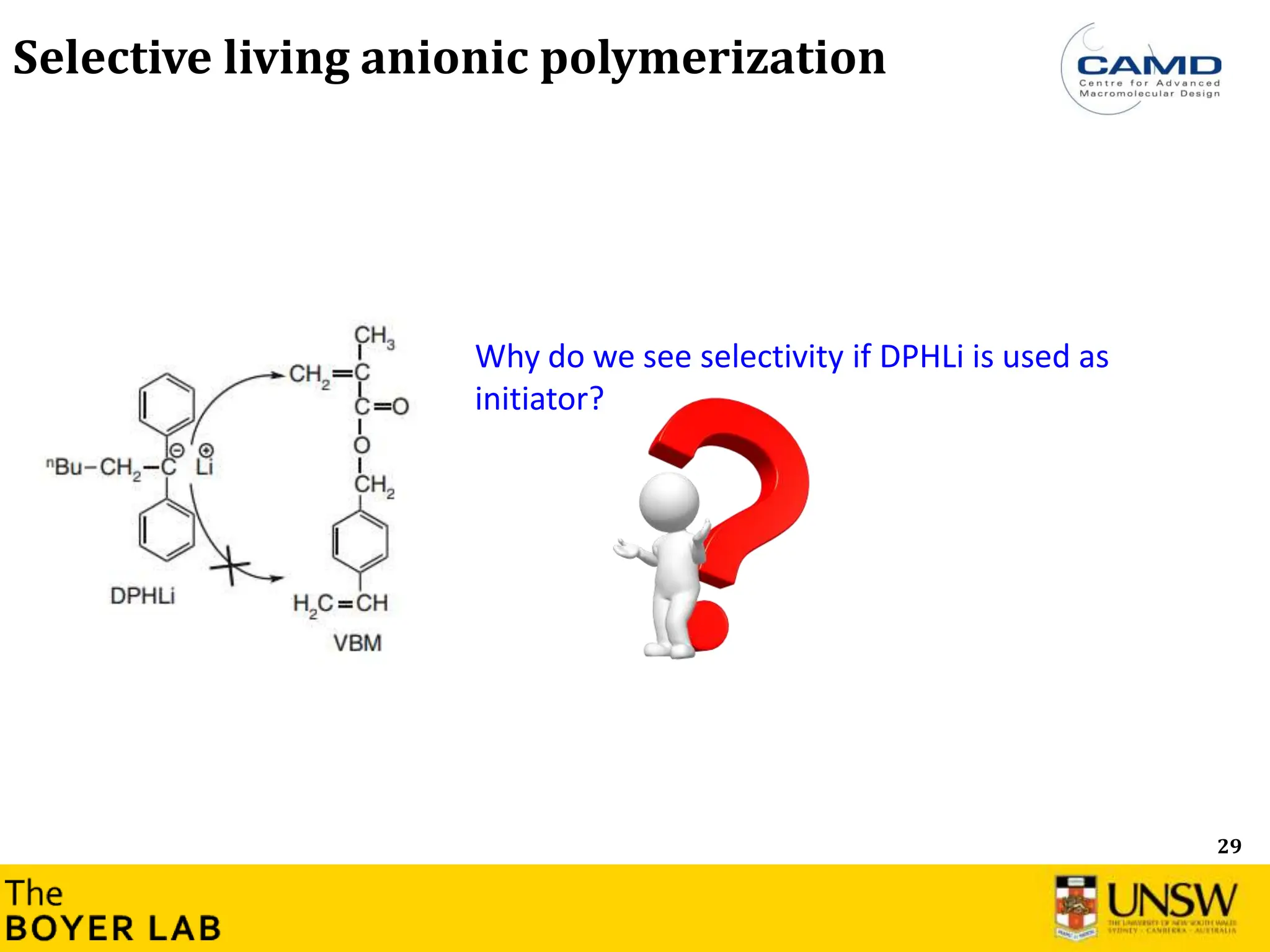
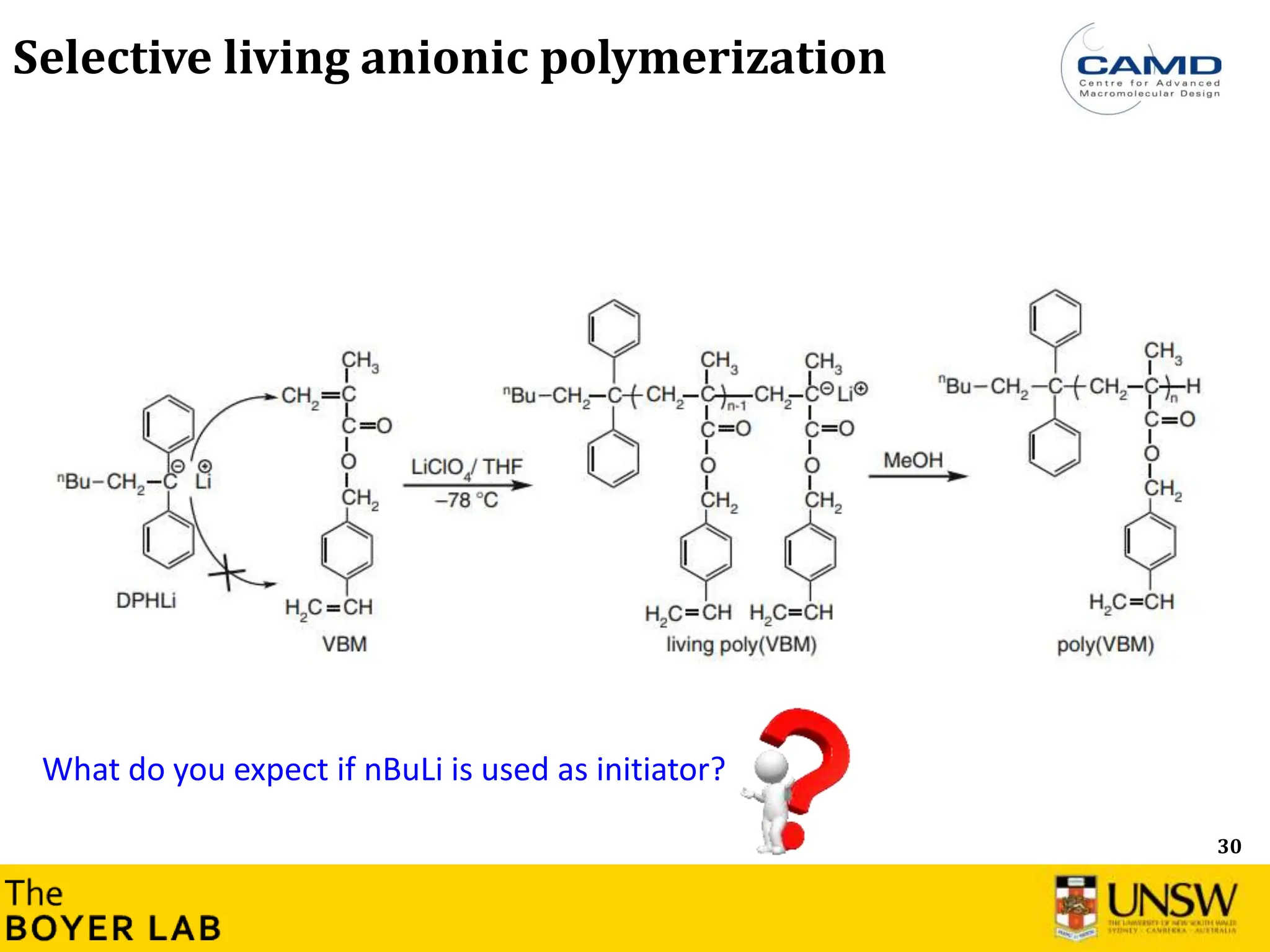

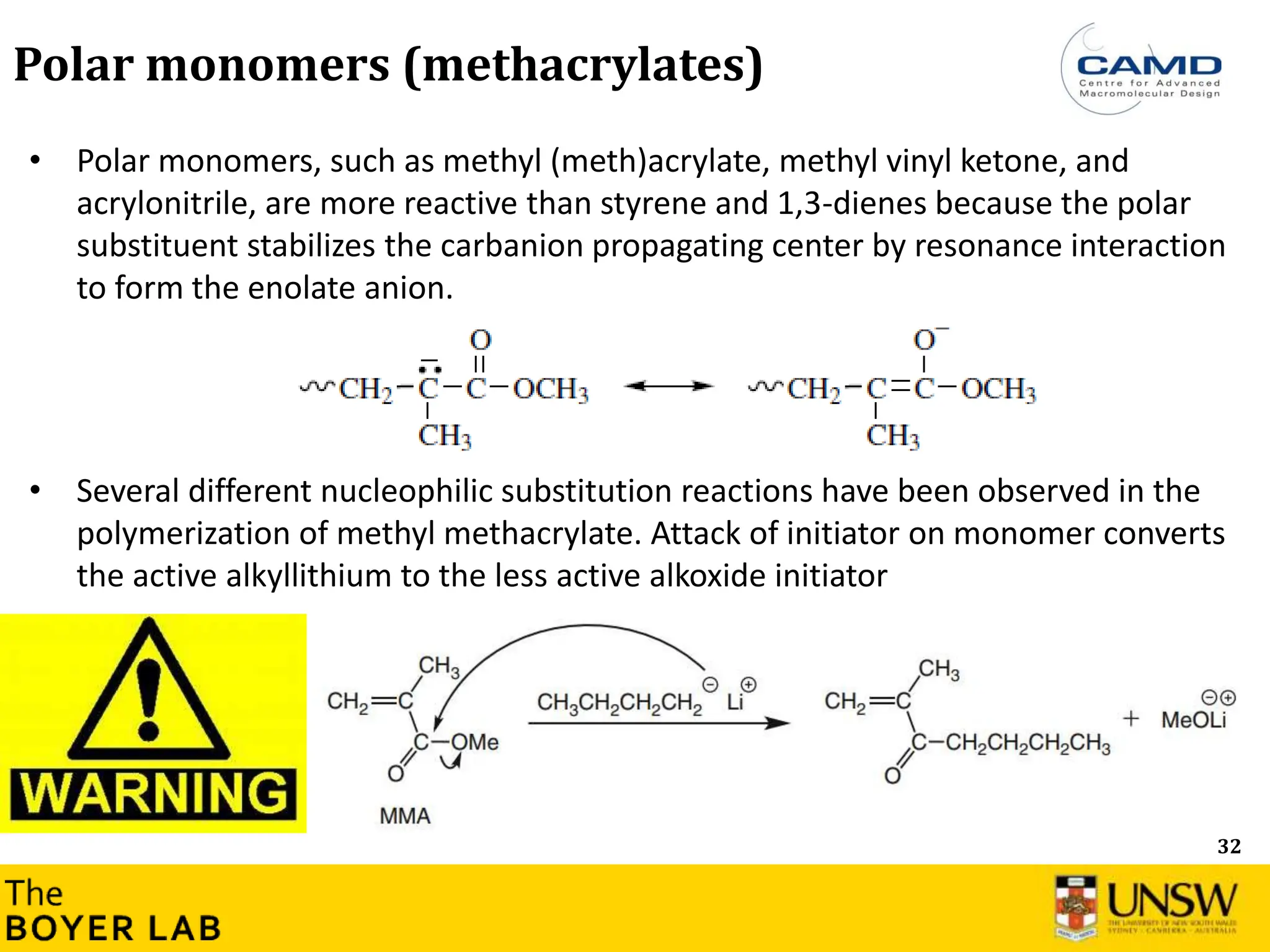
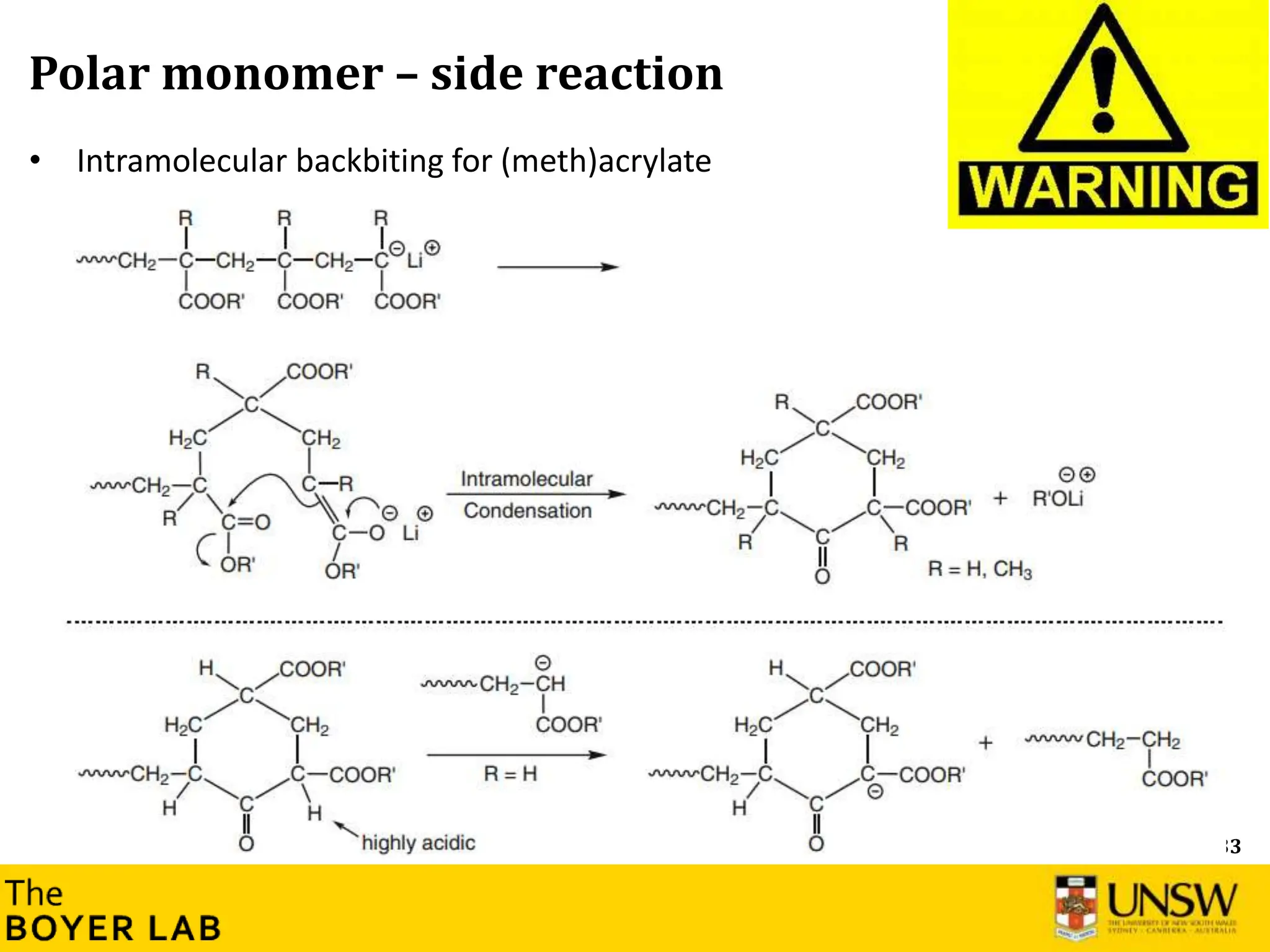
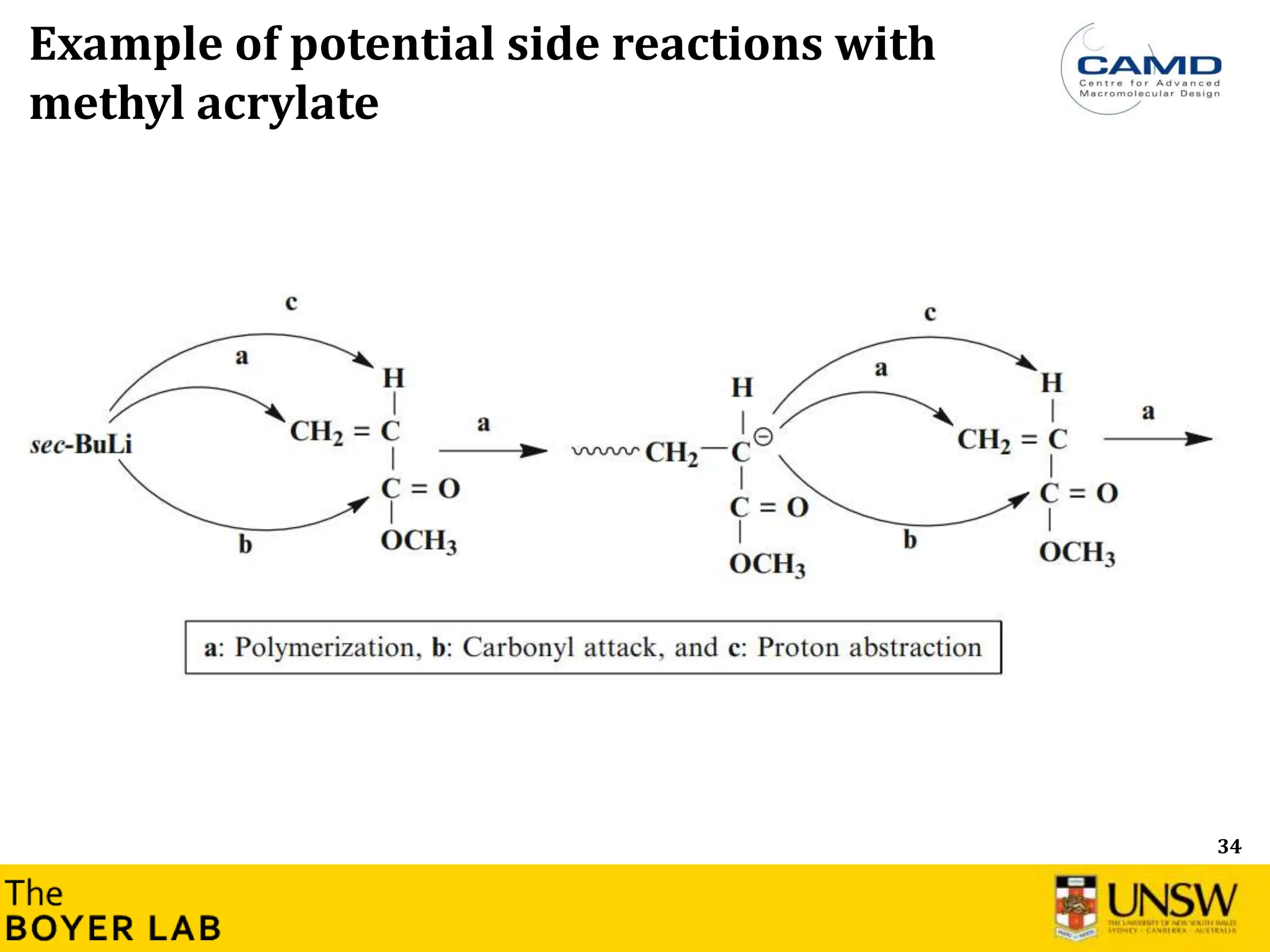
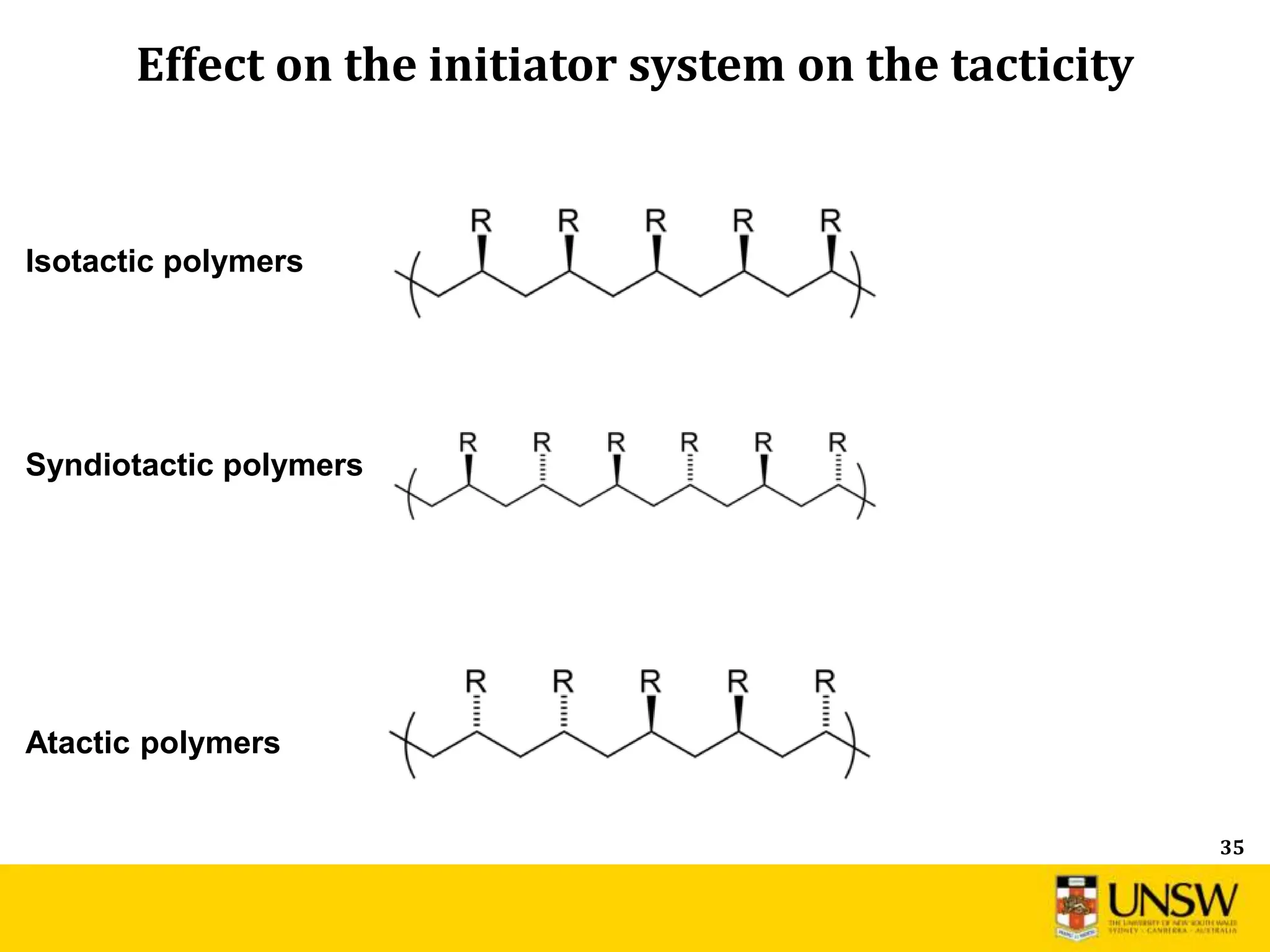
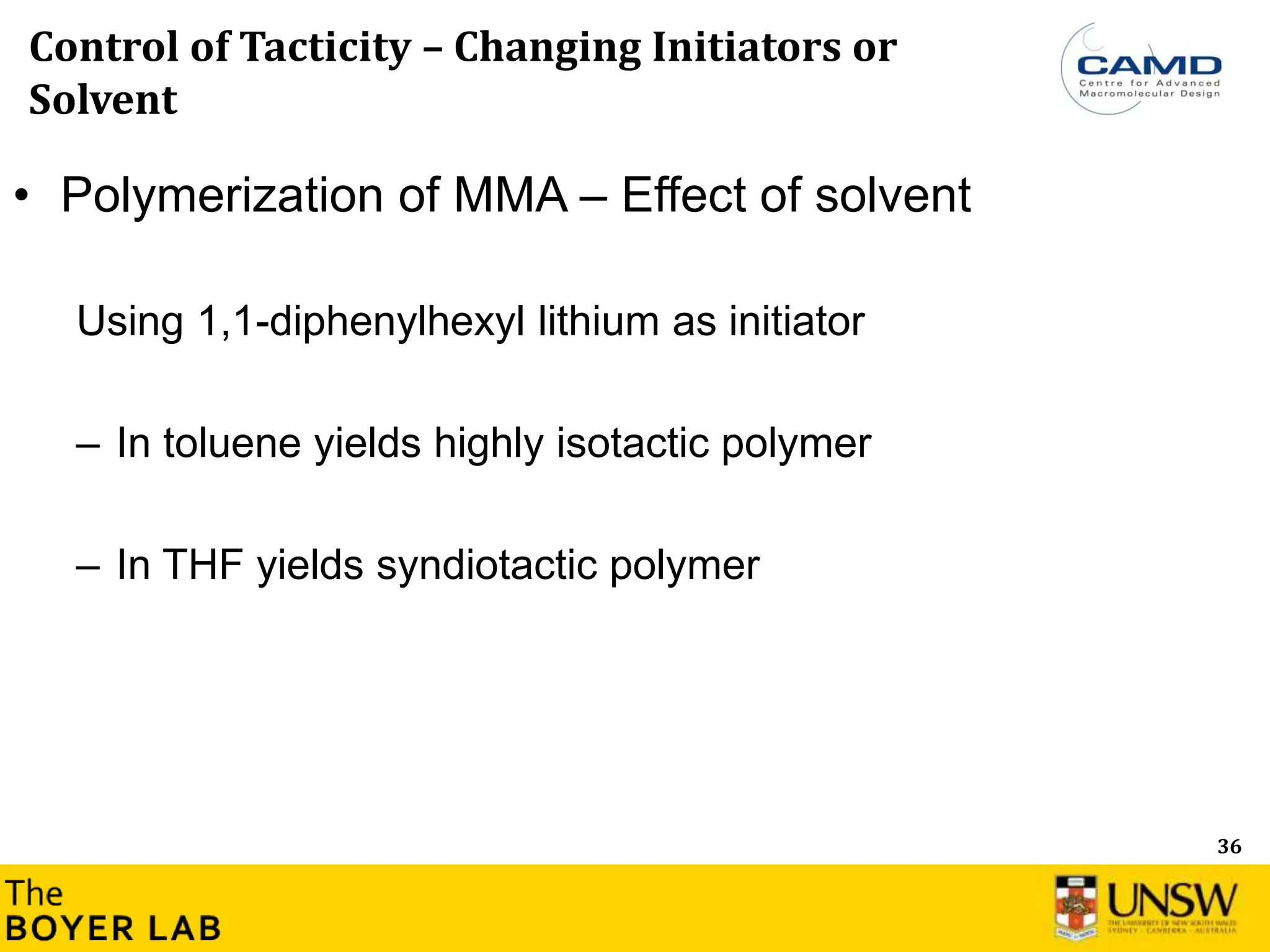
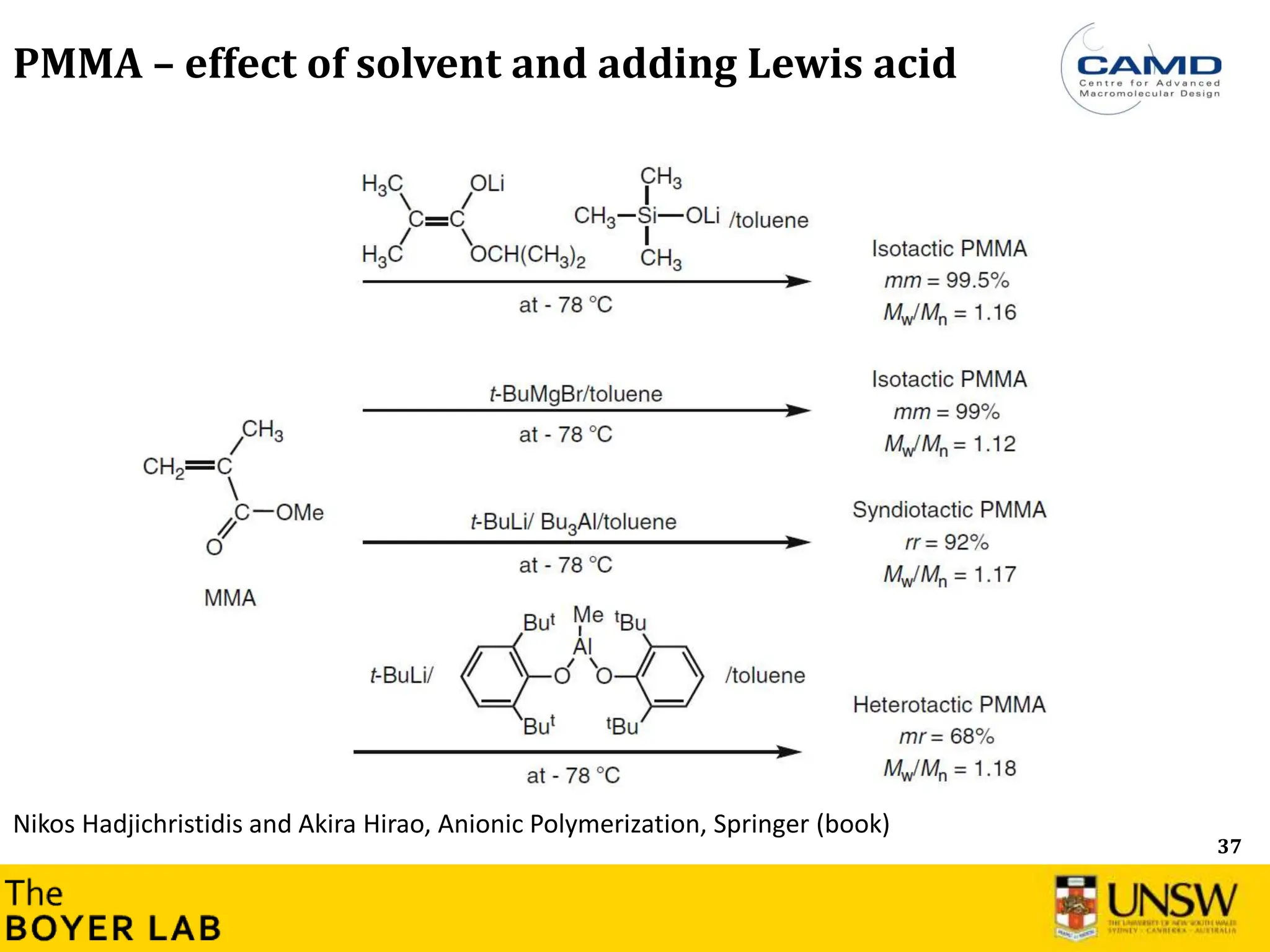
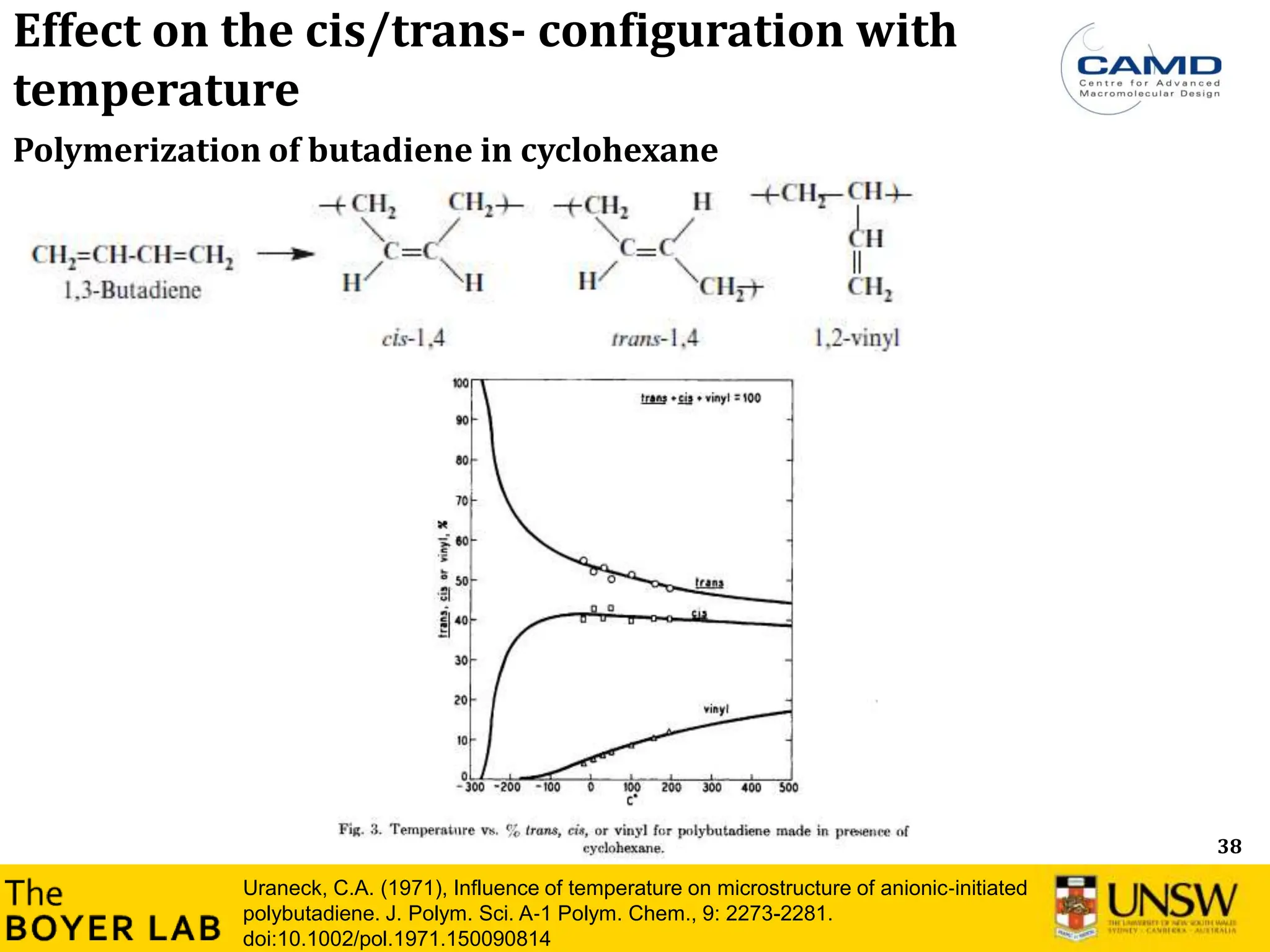
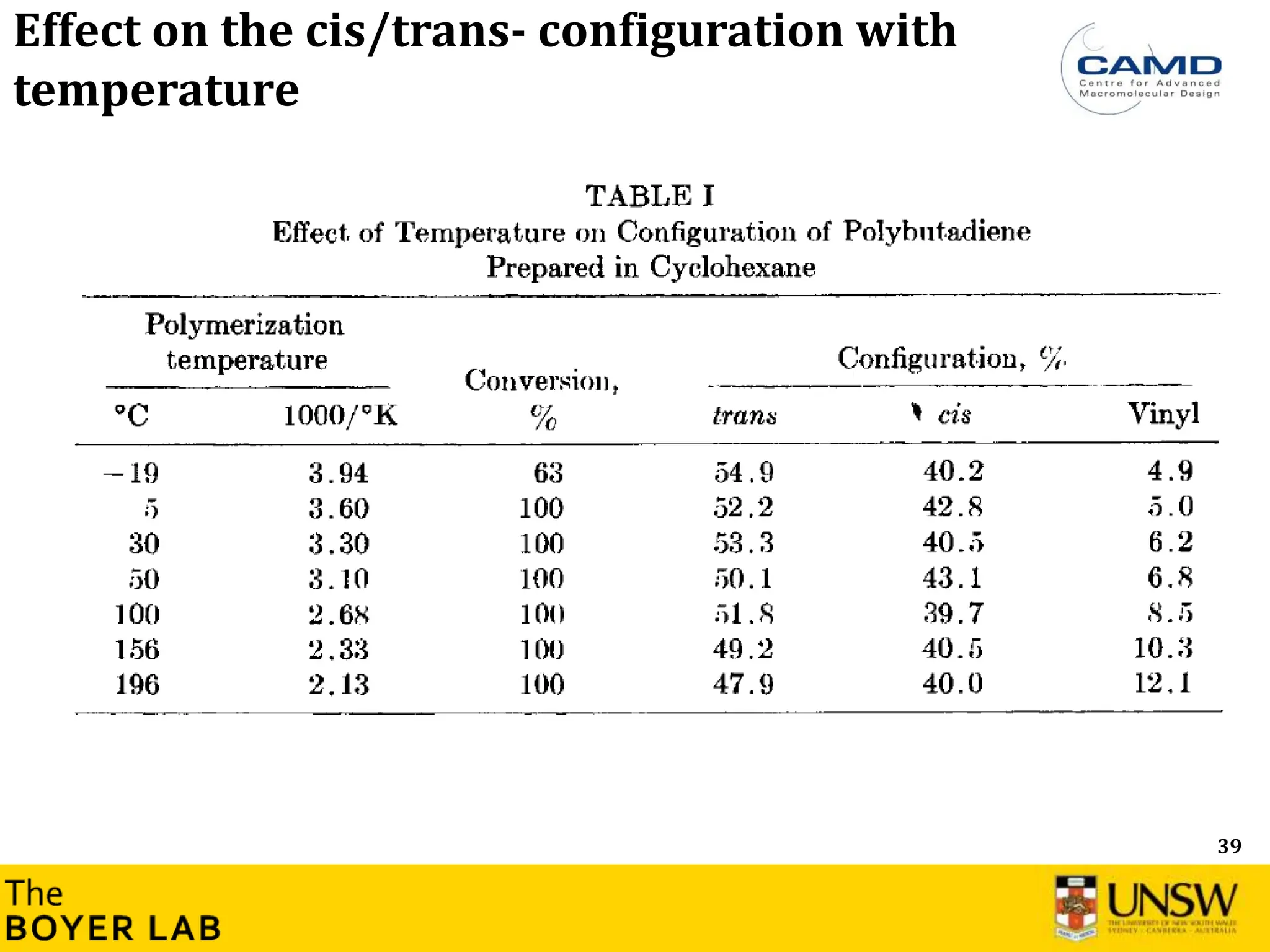
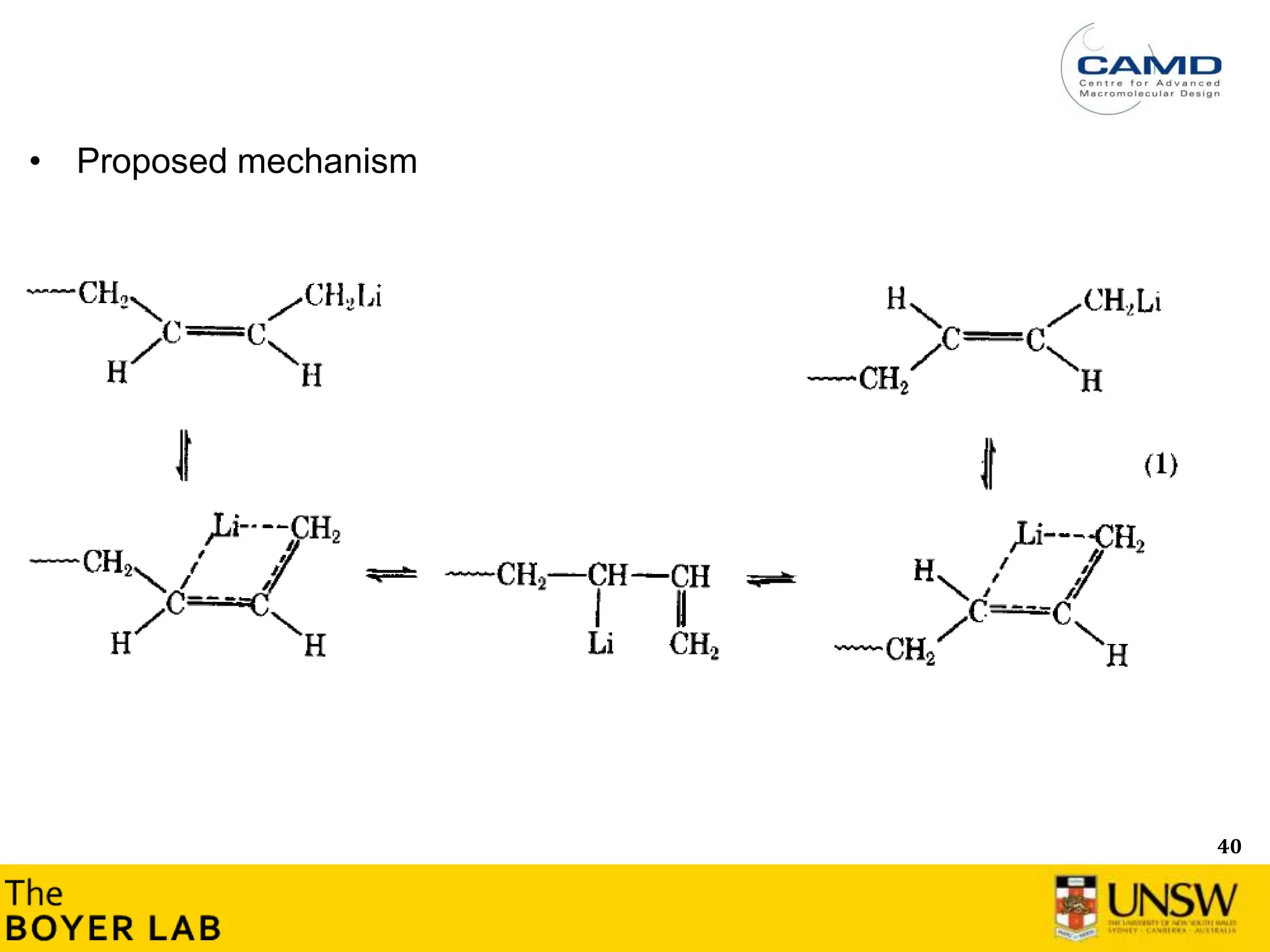
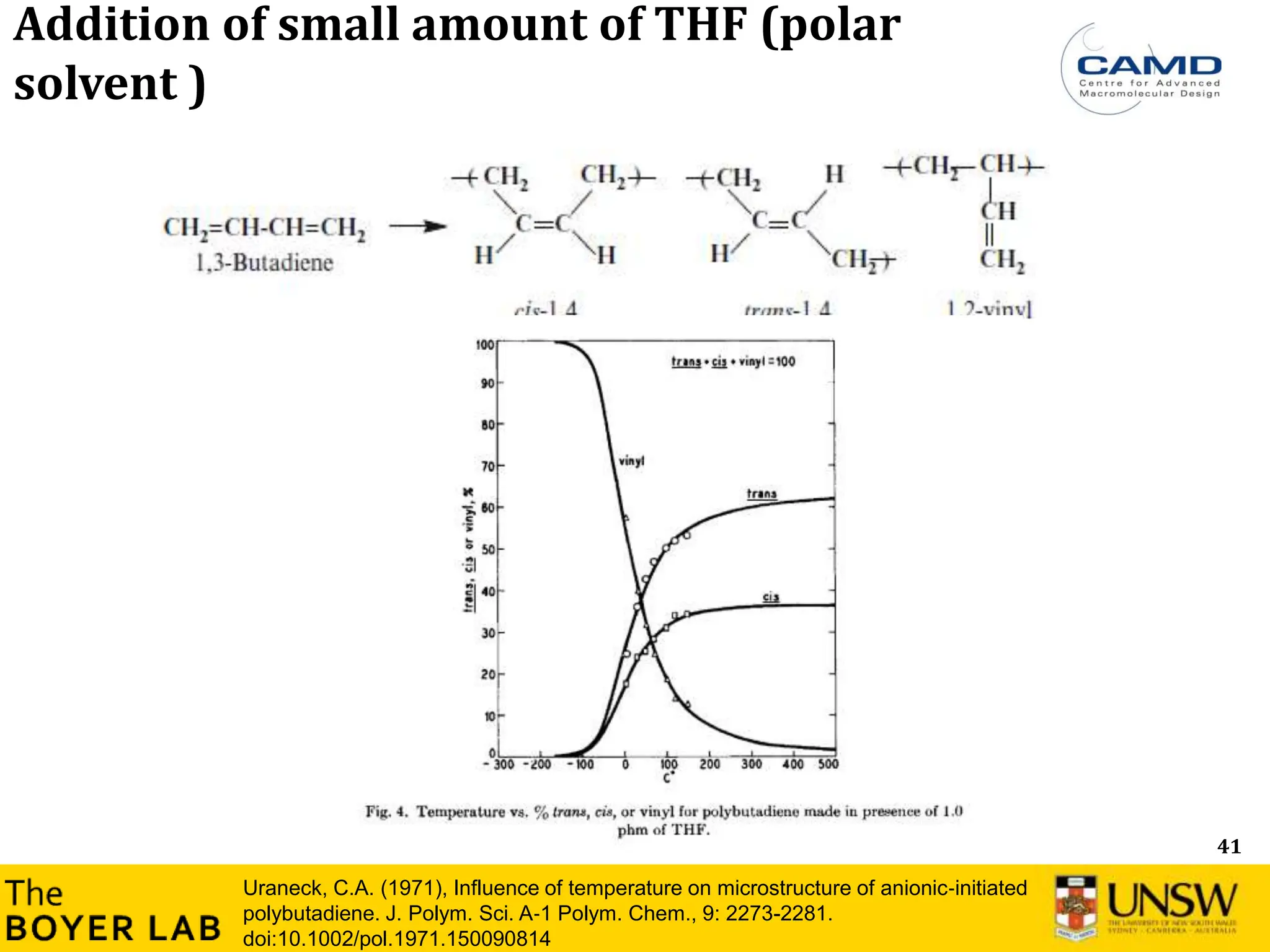
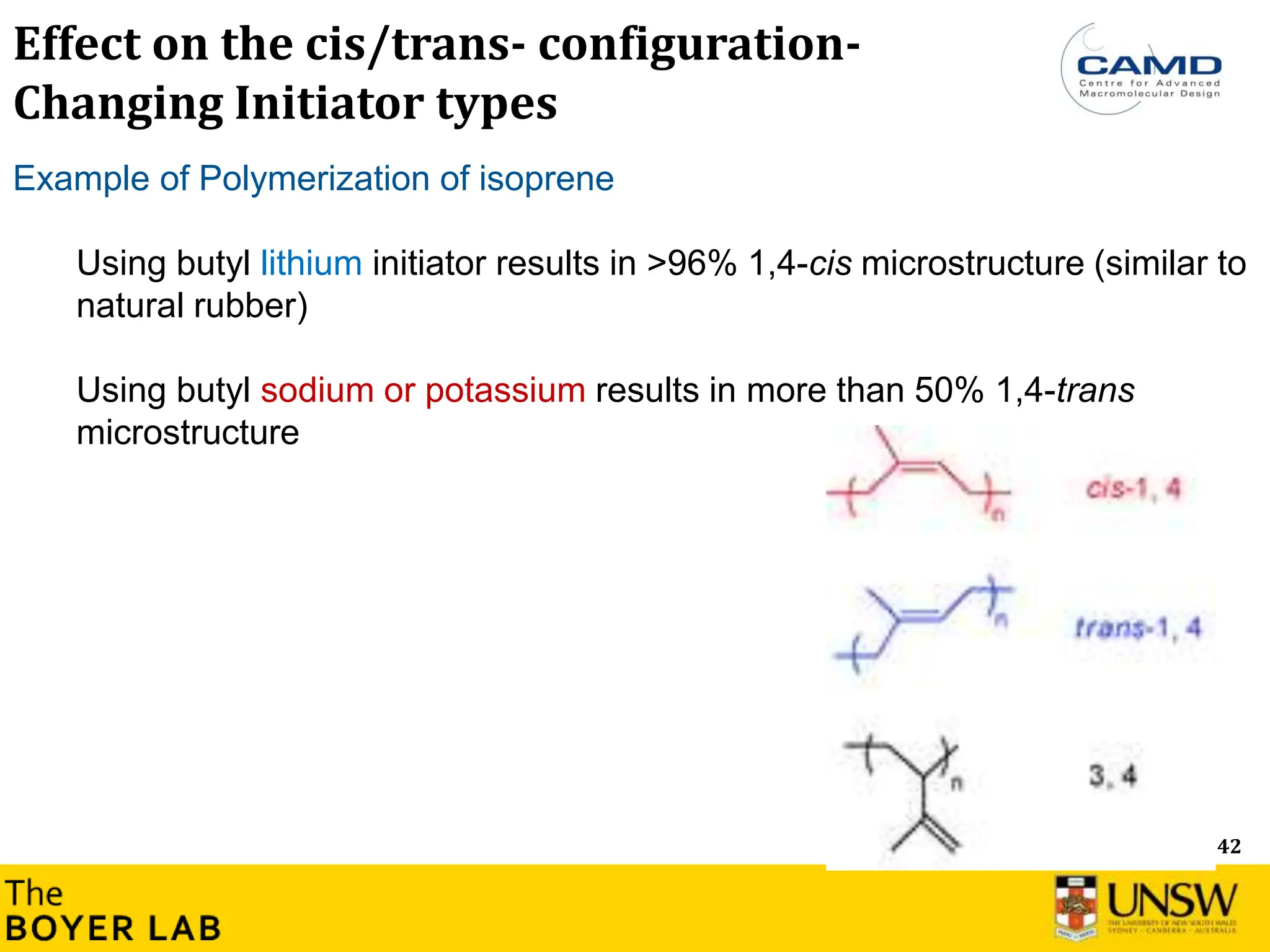


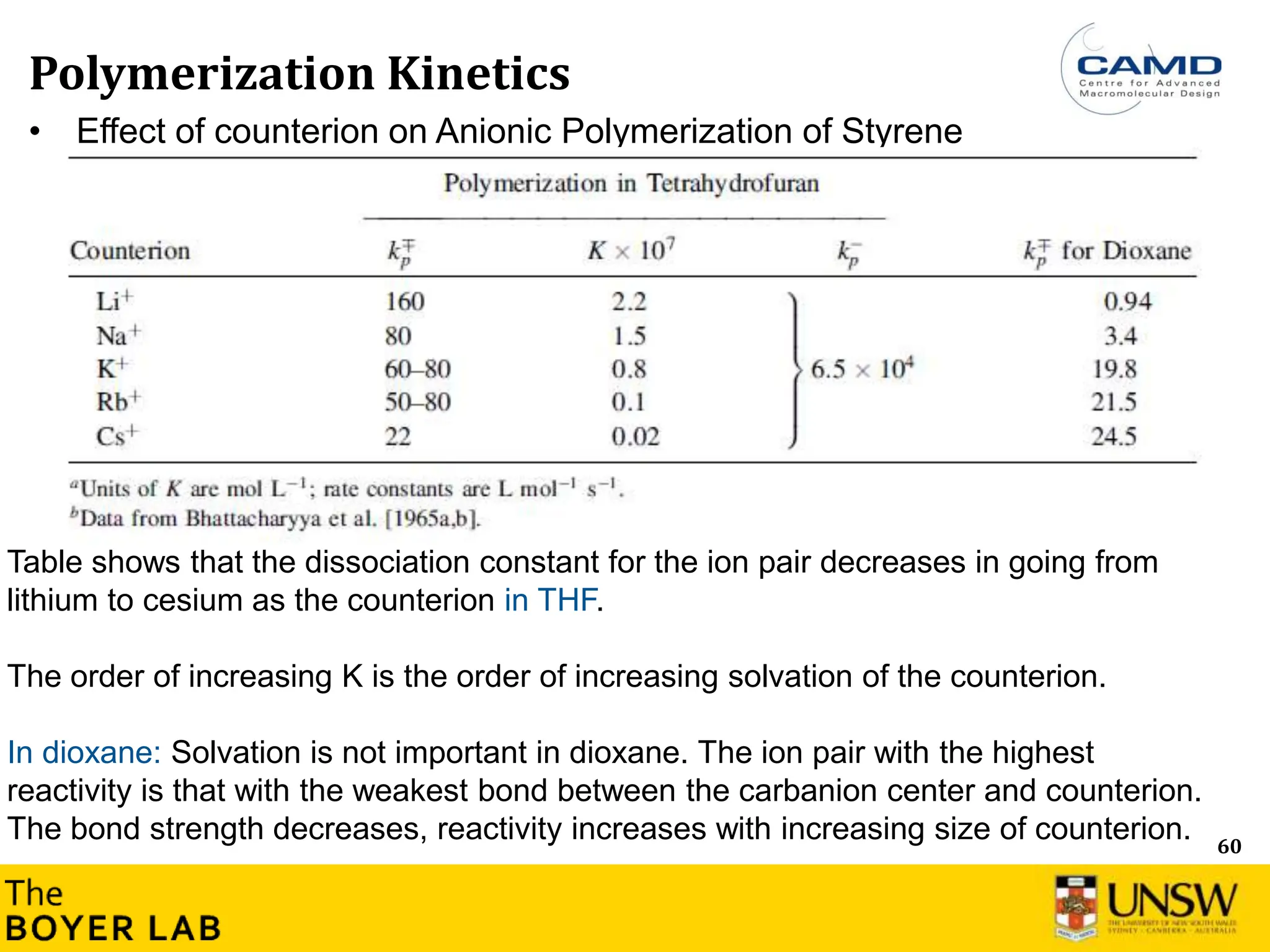
![53
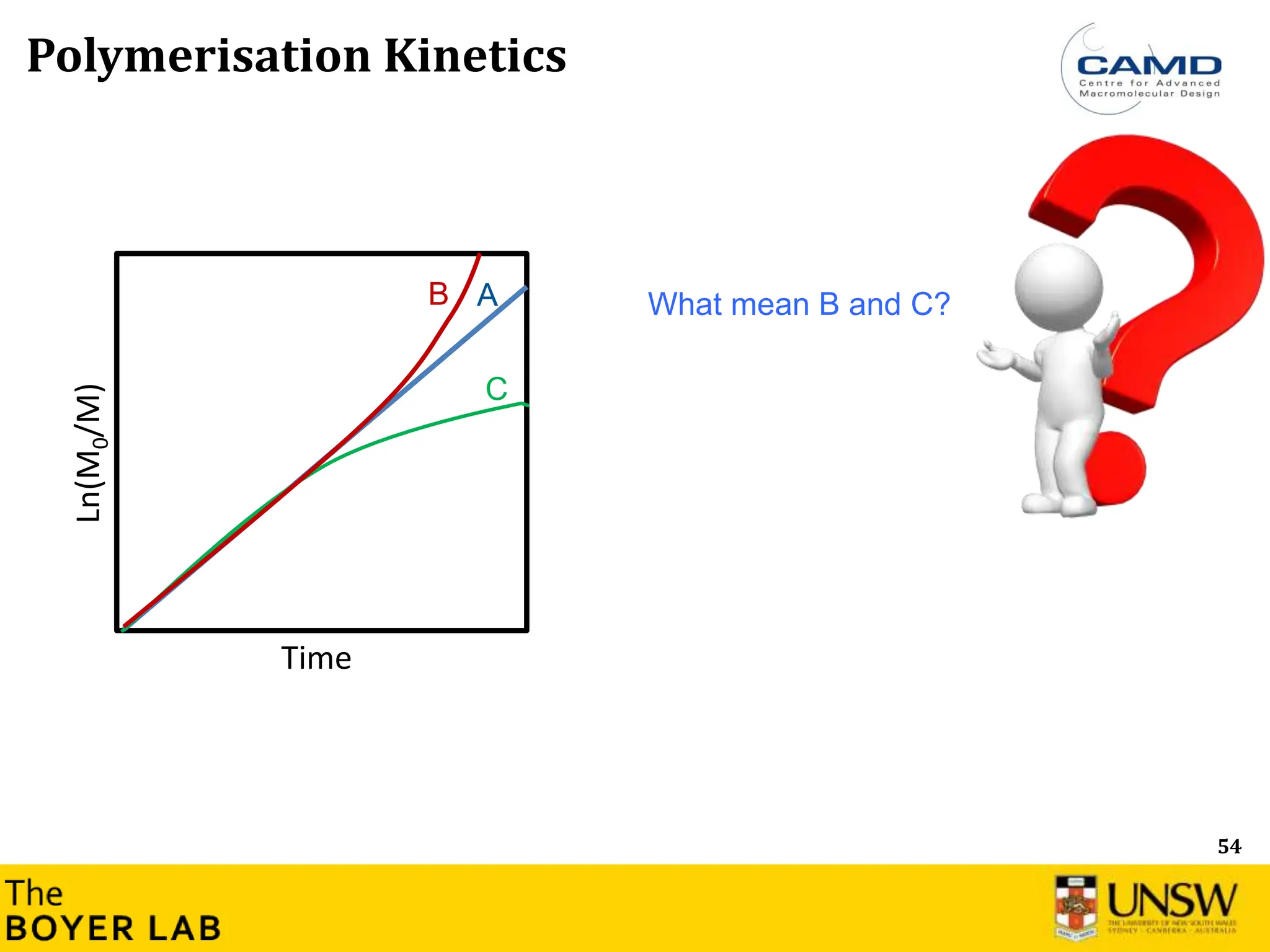
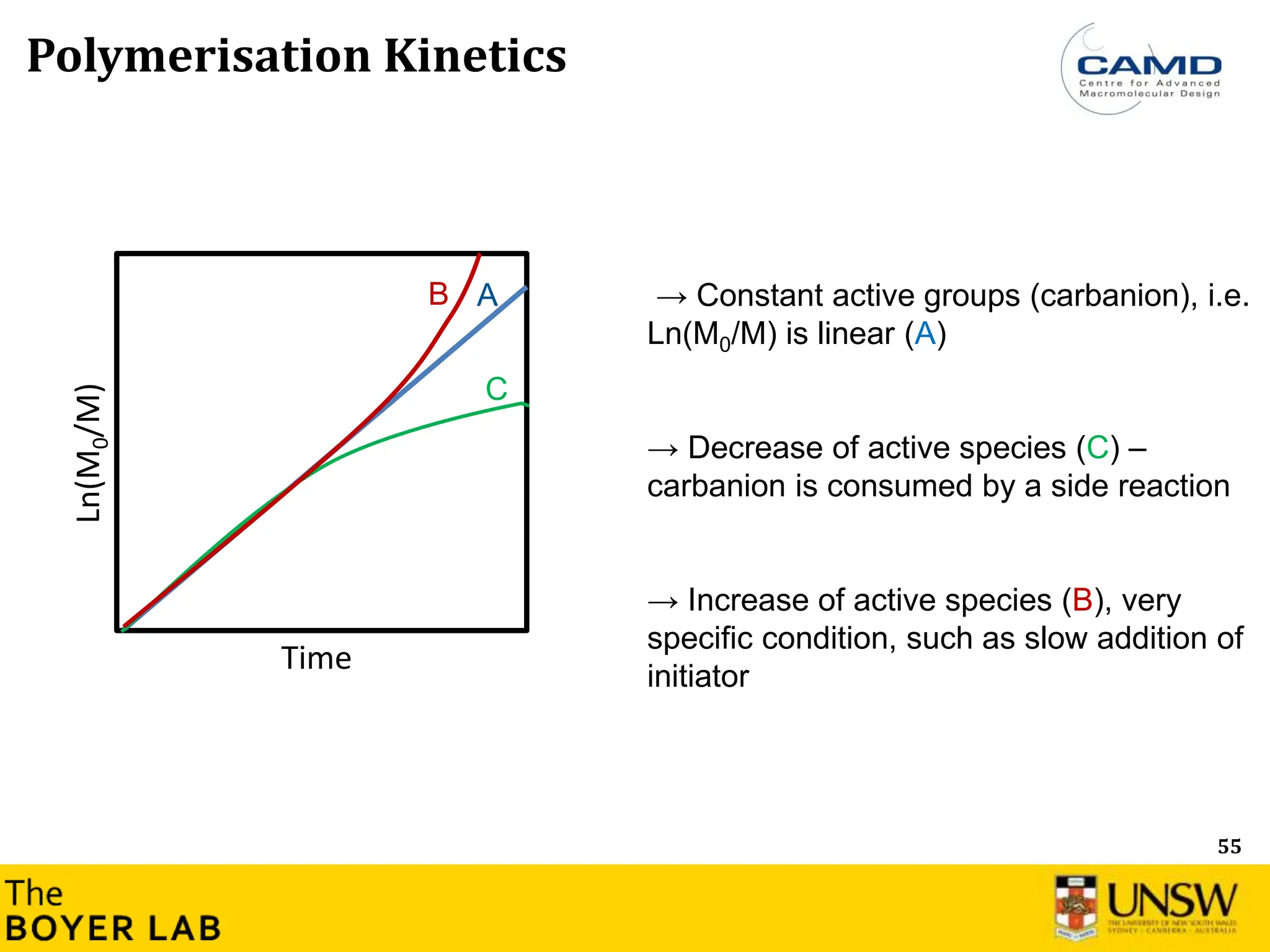
Polymerization Kinetics
Ln([M]
0
/[M]
t
)
Time
Ln([M]0/[M]t) = kp
app × time → Constant concentration of active group
Ln([M]0/[M]t) = Rp= kp
app× [M-] × [M]
[M-] corresponds to active species, where [M] is the total concentration of all types of
living anionic propagating centers (free ions and ion pairs).
→ Most of anionic polymerization: Constant active groups (carbanion), i.e. Ln(M0/M) is
linear](https://image.slidesharecdn.com/anionicpolymerization2024part2-240626111321-d24bca81/75/Anionic-Polymerization-2024-Chemistry-course-53-2048.jpg)
![59
Factors affecting kp
app
• [M-] is combination of [P-] + [P-(C+)] [M-] = [P-] + [P-(C+)]
• Rp = kp
-[P-][M] + Kp
±[P-(C+)][M] (1)
• Rp = = kp
app× [M-] × [M] (2)
Combined (1) and (2)
• kp
app=
[kp
−[P−]+ Kp
±[P−(C+)]
[M−]
• P-(C+) ↔ P- + C+ Dissociation constant: 𝐾 =
𝑃
−
[𝐶
+
]
[𝑃
−
𝐶
+
]
,
• if [P-]=[C+] 𝐾 =
𝑃
−
2
[𝑃
−
𝐶
+
]
𝑃
−
= √(𝐾 × 𝑃
−
𝐶
+
)](https://image.slidesharecdn.com/anionicpolymerization2024part2-240626111321-d24bca81/75/Anionic-Polymerization-2024-Chemistry-course-59-2048.jpg)
![63
Molecular
weight
Monomer conversion
• Linear evolution of Mn versus monomer
conversion
→ Prediction of the molecular weight versus
monomer conversion
Mn = ([M]0/[Initiator]0) × α × MW (Monomer)
+ MW (Initiator)
What is the condition for this equation to be
valid?
Anionic Polymerization – Evolution of Mn
versus monomer conversion](https://image.slidesharecdn.com/anionicpolymerization2024part2-240626111321-d24bca81/75/Anionic-Polymerization-2024-Chemistry-course-63-2048.jpg)
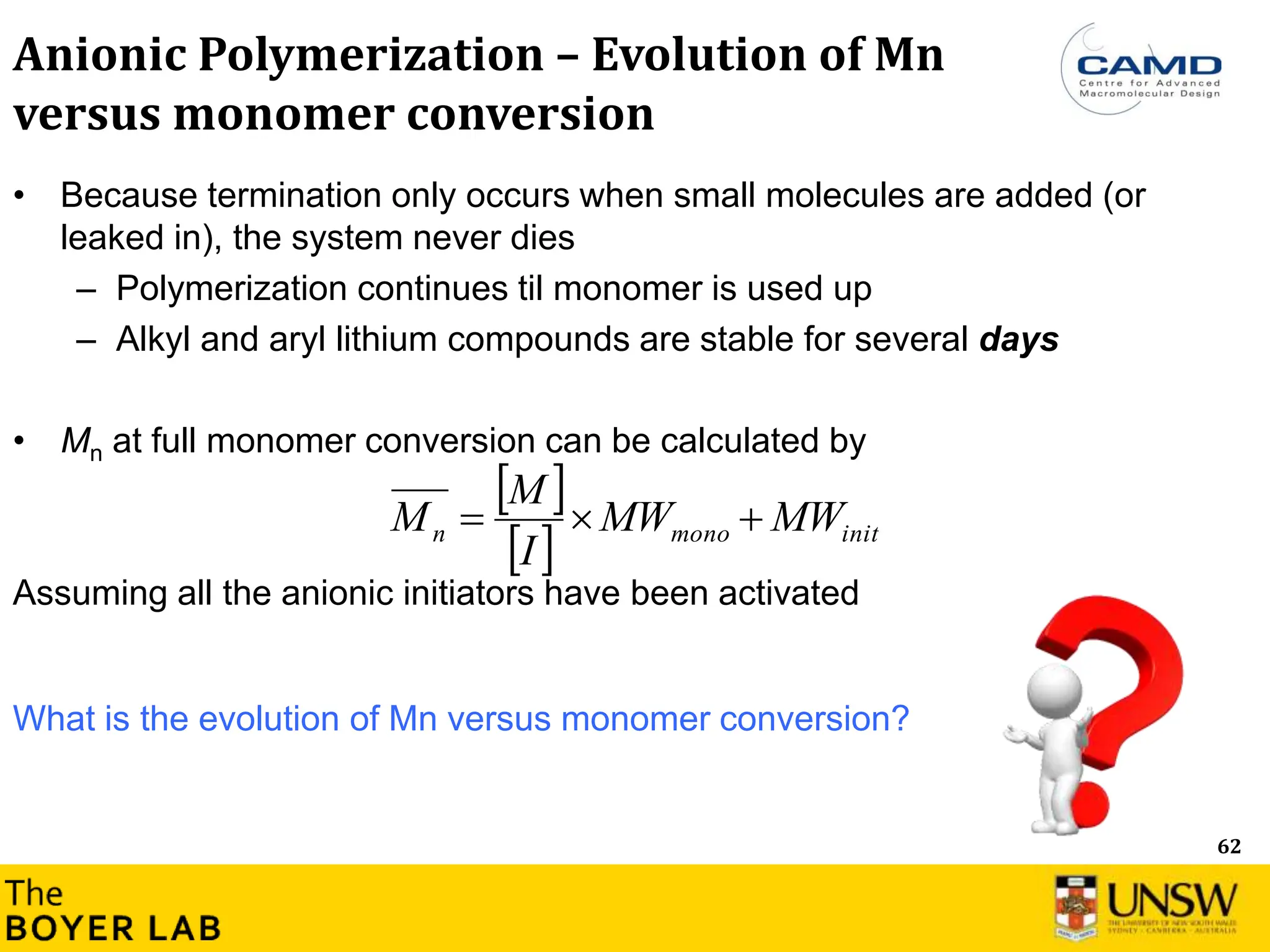
![64
Molecular
weight
Monomer conversion
• Linear evolution of Mn versus monomer
conversion
→ Prediction of the molecular weight versus
monomer conversion
Mn = [M]0/[Initiator]0 × α × MW (Monomer) +
MW (Initiator)
Anionic Polymerization – Evolution of Mn
versus monomer conversion
Condition: In anionic polymerisation, the initiation is fast (all chains are activated at the
beginning of the polymerization) in comparison to the rate of chain propagation.
What is the consequence for the dispersity (or polydispersity)?](https://image.slidesharecdn.com/anionicpolymerization2024part2-240626111321-d24bca81/75/Anionic-Polymerization-2024-Chemistry-course-64-2048.jpg)