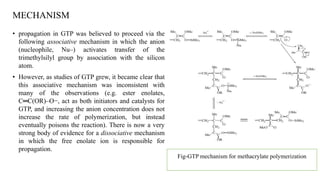
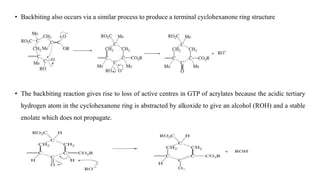
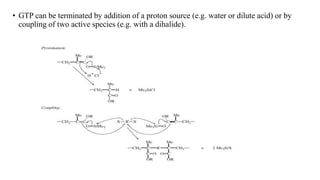
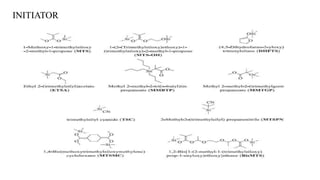
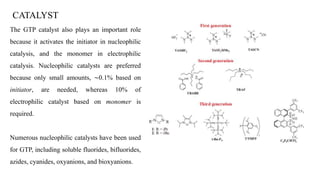
Group transfer polymerization (GTP) is a "quasi-living" polymerization technique that allows controlled polymerization of monomers like methacrylates. GTP uses silyl ketene acetal initiators and metal-free catalysts. Propagation occurs via Michael addition, transferring the silyl group to the added monomer. While initially thought to proceed via an associative mechanism, evidence supports a dissociative mechanism. GTP produces polymers with controlled molecular weight and can create block copolymers, random copolymers, and other architectures. Applications include dispersing agents for water-based inks.