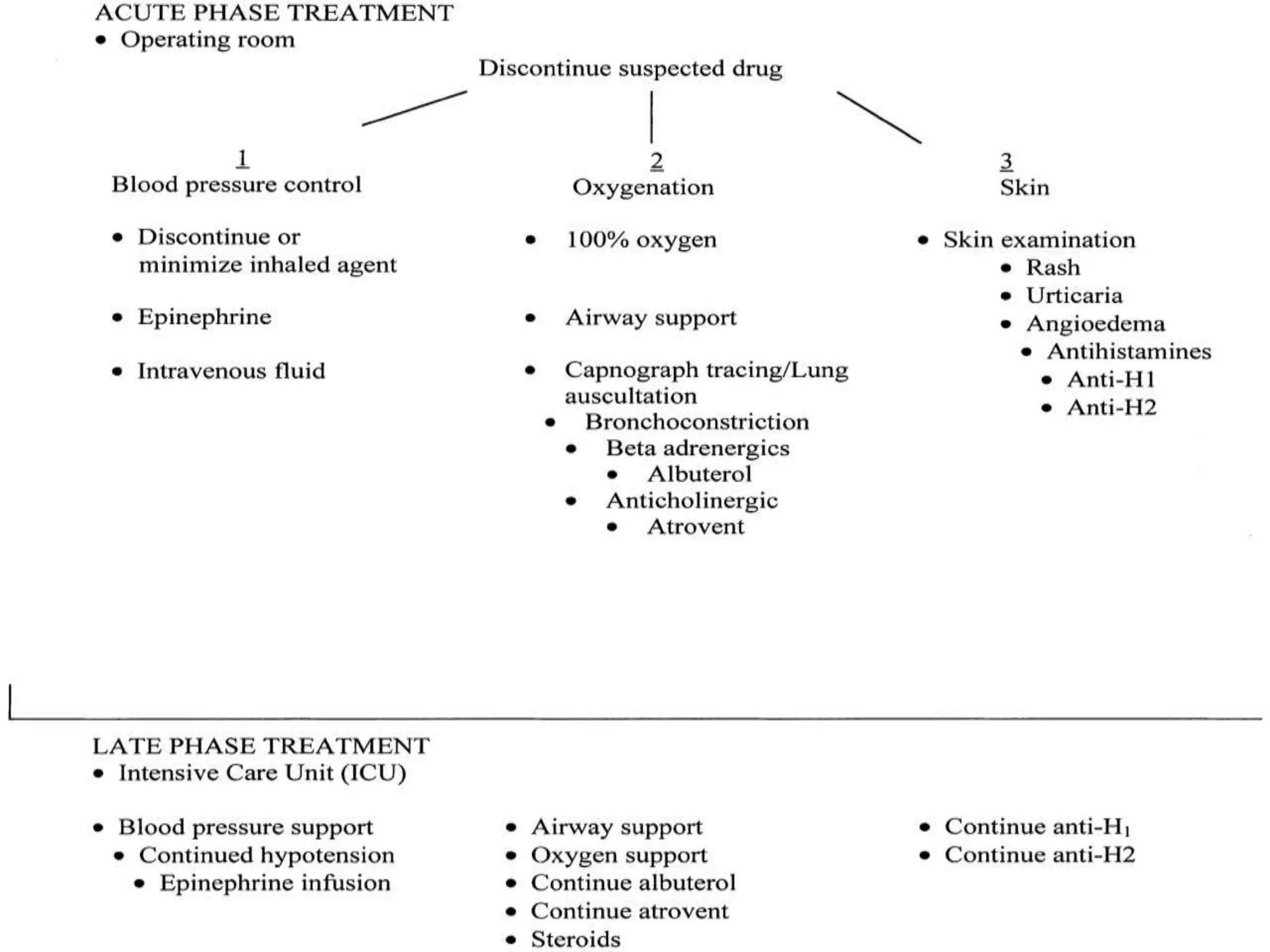
Anaphylaxis is a severe allergic reaction that affects multiple body systems. It can be caused by medications like muscle relaxants, antibiotics, and opioids during surgery. Common symptoms include low blood pressure, rashes, and breathing difficulties. Muscle relaxants are the most frequent cause of anaphylaxis during procedures. While rare, allergic reactions to substances like latex gloves, egg products in propofol, and preservatives in local anesthetics are also possible. Testing can identify specific allergies to help select safer alternatives for future medical care.