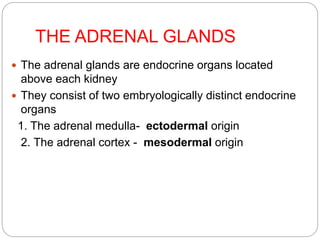
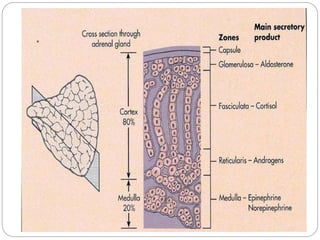
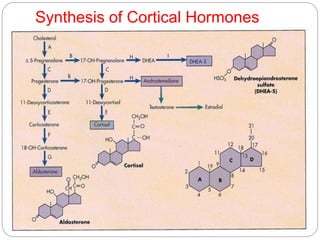
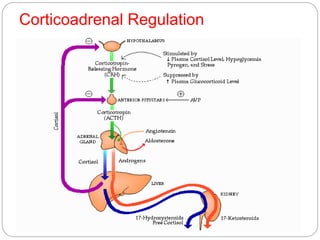
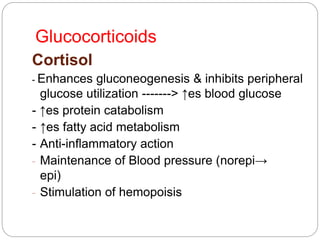
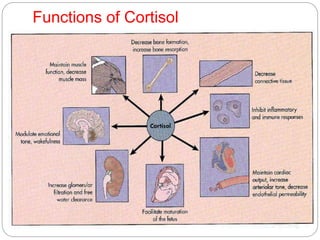
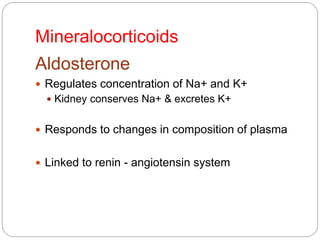
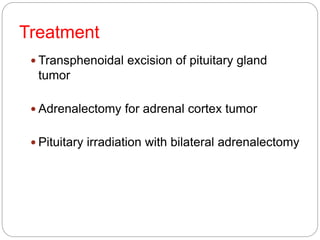
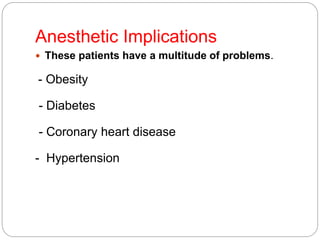
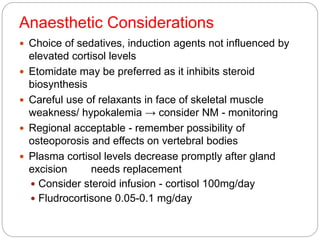
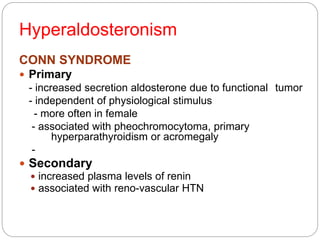
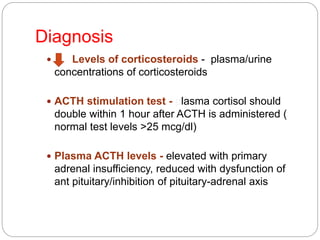
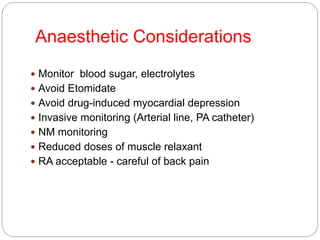
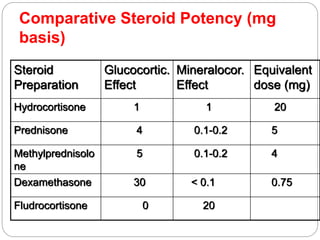
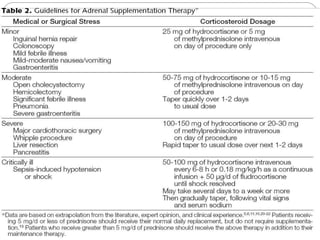
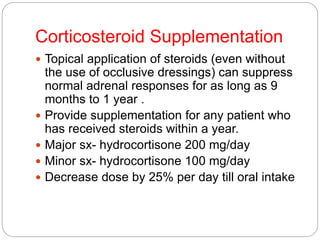
This document discusses the anatomy, physiology, and pathological disorders of the adrenal glands and their management during anaesthesia. It describes the adrenal cortex and medulla, hormone production and regulation. Pathologies covered include Cushing's syndrome, Conn's syndrome, hypoaldosteronism, and primary/secondary adrenal insufficiency. Perioperative management focuses on fluid/electrolyte balance, stress dosing of steroids, and monitoring for adrenal crises. Determining return of normal adrenal function and intensive care management are also outlined.