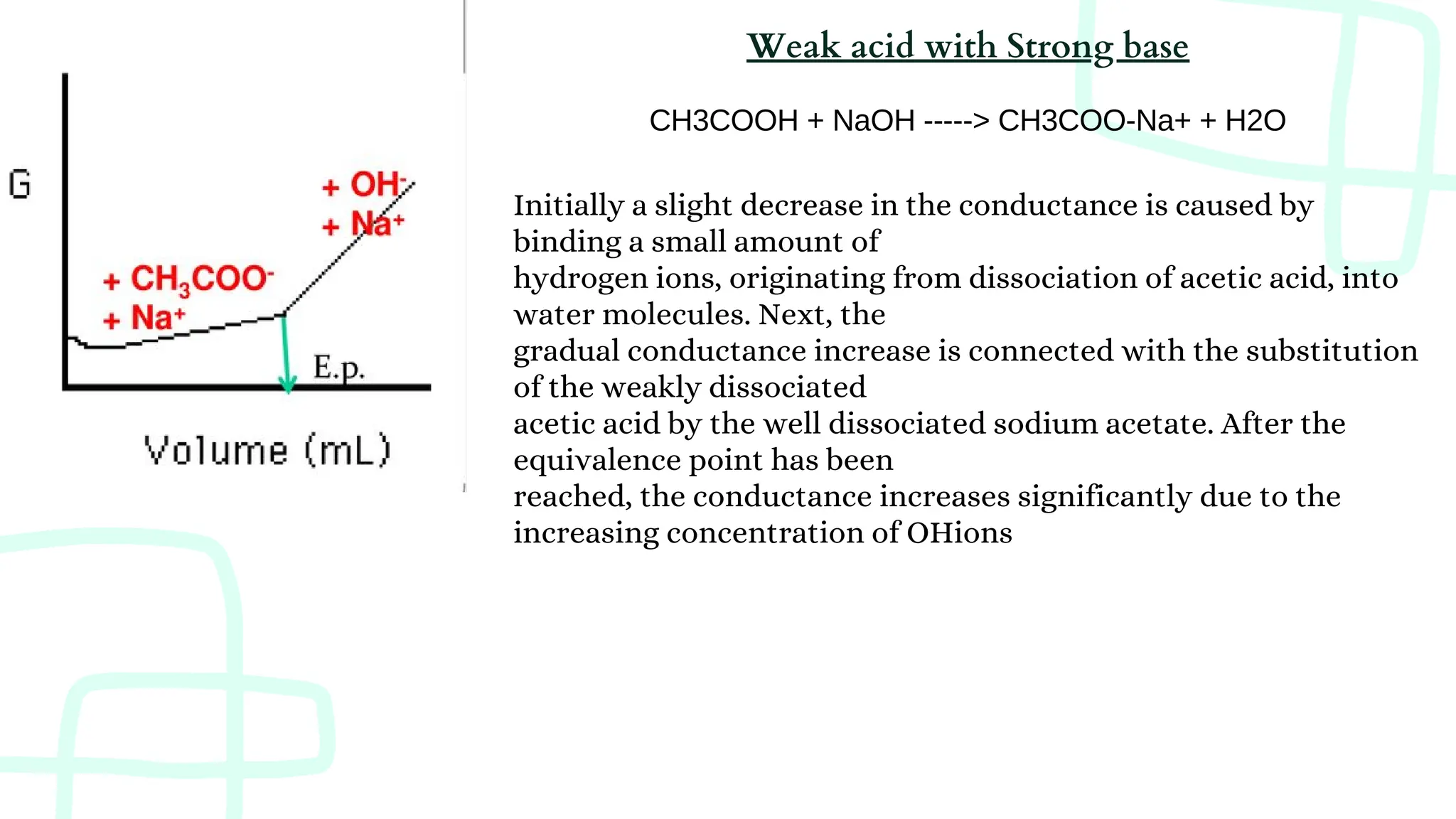
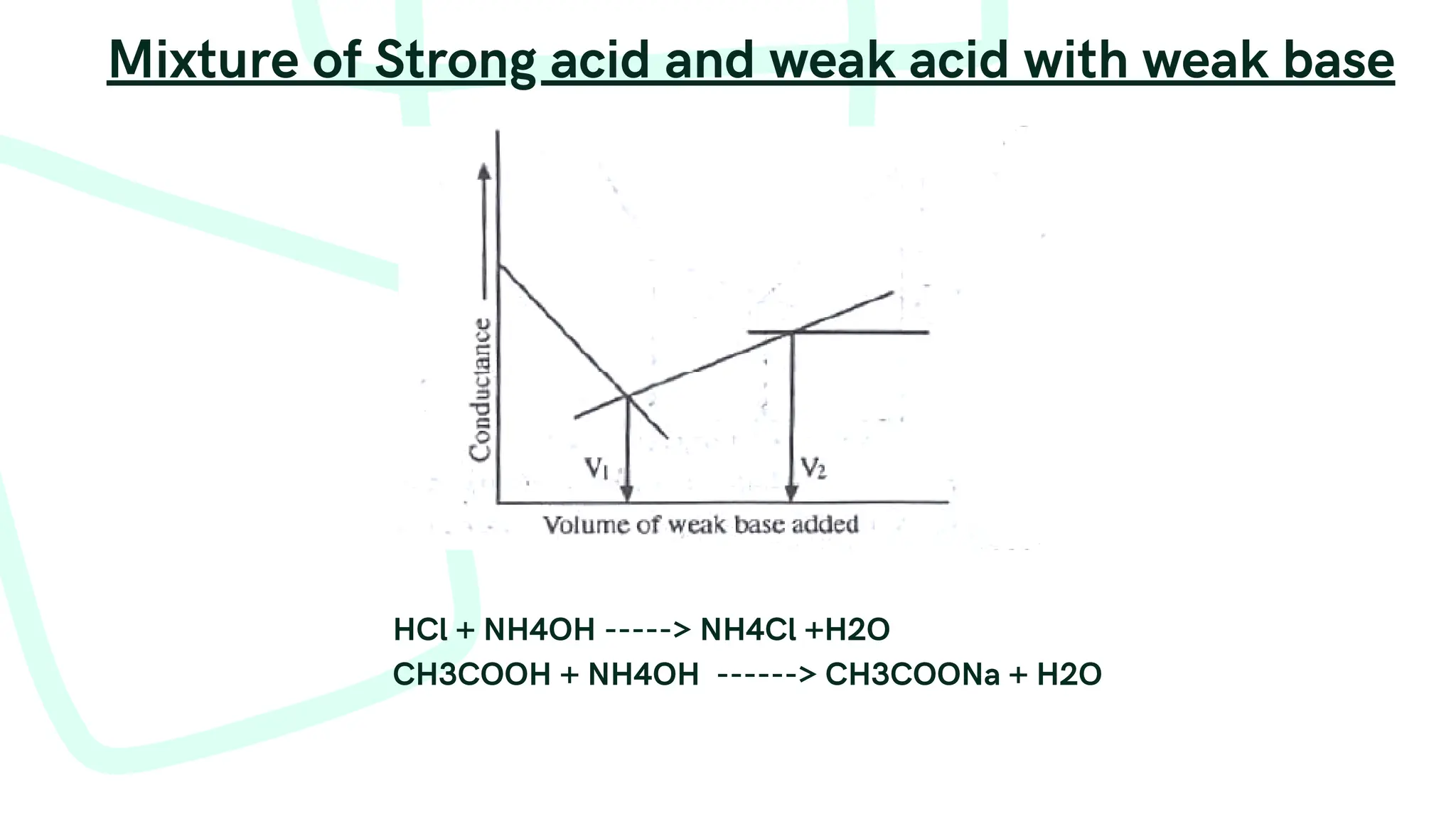
The document discusses photometric and conductometric titration techniques, outlining their principles, procedures, and applications. It highlights the importance of absorbance and conductance changes during titration to determine equivalence points and emphasizes the conditions affecting these measurements. Limitations of each method are also addressed, particularly in relation to concentration ranges and the presence of interfering species.