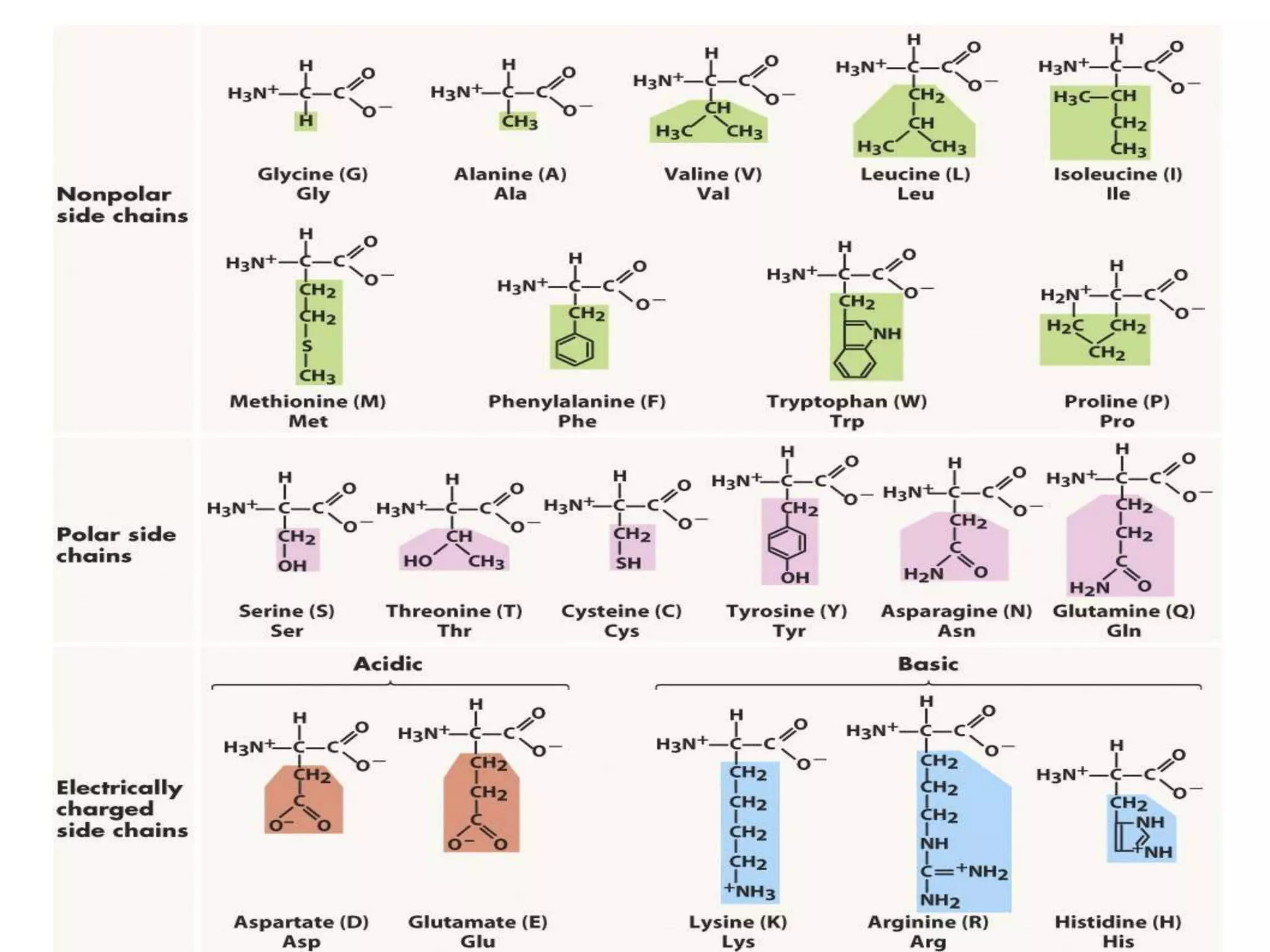
This document discusses amino acids and proteins. It defines that amino acids are the monomer units that make up proteins and polypeptides. There are 300 amino acids found in nature but only 20 are used in protein synthesis. Proteins perform important structural, catalytic and regulatory functions in the body. The structure of proteins involves four levels: primary, secondary, tertiary and quaternary. Denaturation involves the unfolding of protein structure through physical or chemical means.



![• Short polymer of amino acid [polypeptides] perform
prominent role in neuro-endocrine system as
hormones, hormone releasing factor, neuro-
modulators or neurotransmitters.
• Genetic defect in metabolism of amino acids can
result in severe illness such as
– Phenyl ketonuria,
– Albinism,
– Maple syrup urine disease etc.
• Amino aciduria, another genetic disease result from
an impaired ability to transport specific amino acid
to cell](https://image.slidesharecdn.com/aminoacids20-200223133901/75/Amino-acid-Protein-4-2048.jpg)
![Structure
• Each amino acid [except proline]
has
– A carboxyl group
– An amino group
– A side chain (R)
Bonding to α carbon
• At physiologic pH (7.4) carboxyl
group is dissociated (COO⁻) &
amino group is protonated (NH₃⁺)
• Proline- side chain & α amino group
form a ring structure [imino group]
a](https://image.slidesharecdn.com/aminoacids20-200223133901/75/Amino-acid-Protein-5-2048.jpg)


![Essential amino acid
• Ten of 20 amino acid needed for synthesis of body
protein are essential
• They can not be synthesized in human at adequate rate
• So must be supplied in diet
• 8 are essential all the time
• Arginine & histidine: required only in period of rapid
tissue growth [childhood & recovery from illness]](https://image.slidesharecdn.com/aminoacids20-200223133901/75/Amino-acid-Protein-9-2048.jpg)

![• Zwitter ion: Dipolar form of a molecule which
contain same amount of +ve & -ve ion. At pI,
amino acid become zwitter ion
– COOH looses H⁺ [COO⁻], -NH₂ gains H⁺ [NH₃⁺]
Diprotonated form Deprotonated formDipolar form](https://image.slidesharecdn.com/aminoacids20-200223133901/75/Amino-acid-Protein-11-2048.jpg)

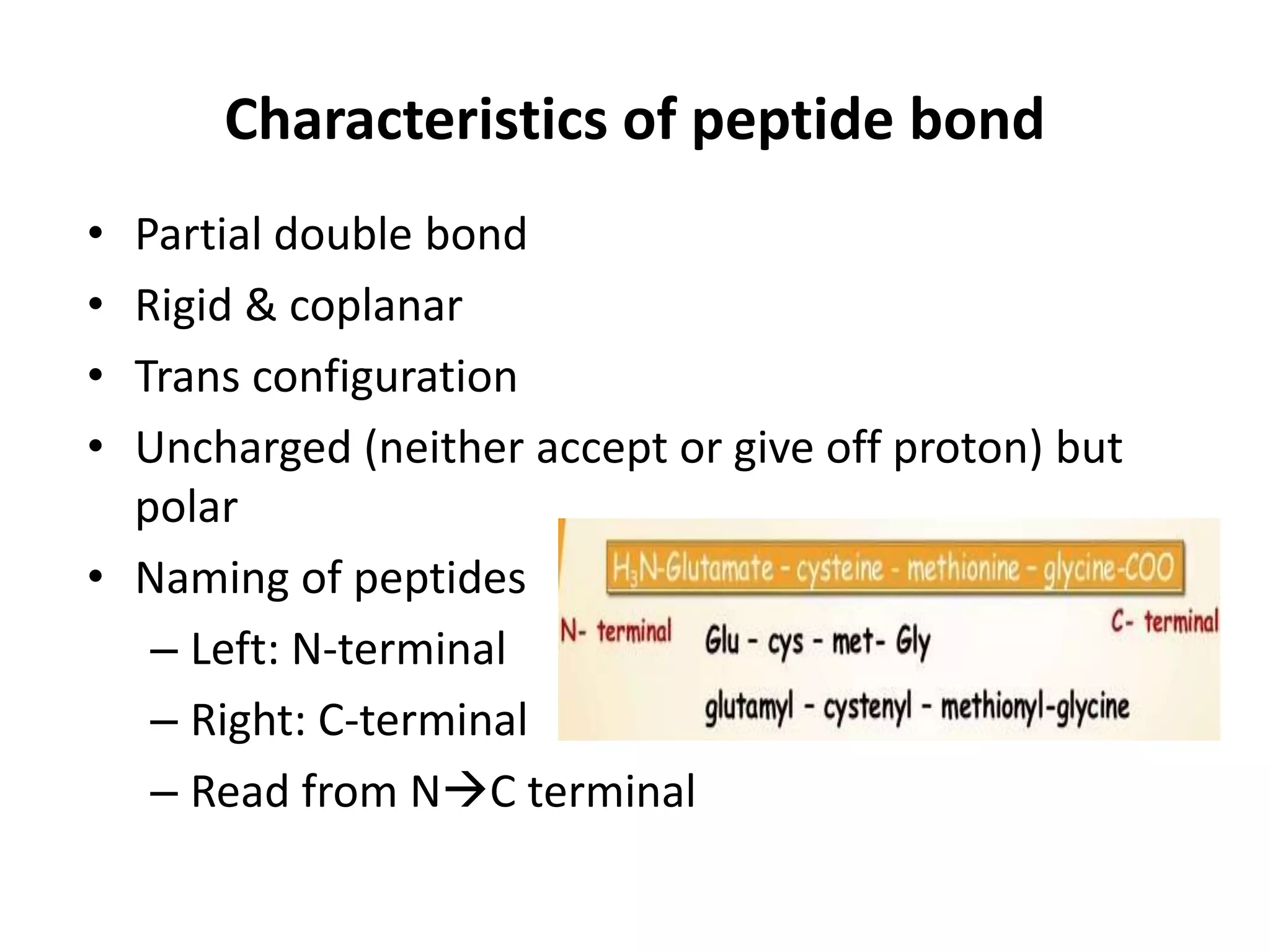











![Denaturation
• Unfolding & disorganization or nonspecific
alteration in the secondary, tertiary &
quaternary structure of protein molecule
• Primary structure is not altered [no hydrolysis
of peptide bond]
• Results in
– ↓ solubility
– ↑ precipitability
– May lose biological activity](https://image.slidesharecdn.com/aminoacids20-200223133901/75/Amino-acid-Protein-25-2048.jpg)

![Denaturation
Denaturing agents are-
• Heat
• Organic solvent [Urea]
• Mechanical mixing
• Strong acid/ base
• Detergents
• Ions of heavy metal [Hg, Pb]
• X-ray, UV ray, high pressure](https://image.slidesharecdn.com/aminoacids20-200223133901/75/Amino-acid-Protein-27-2048.jpg)

