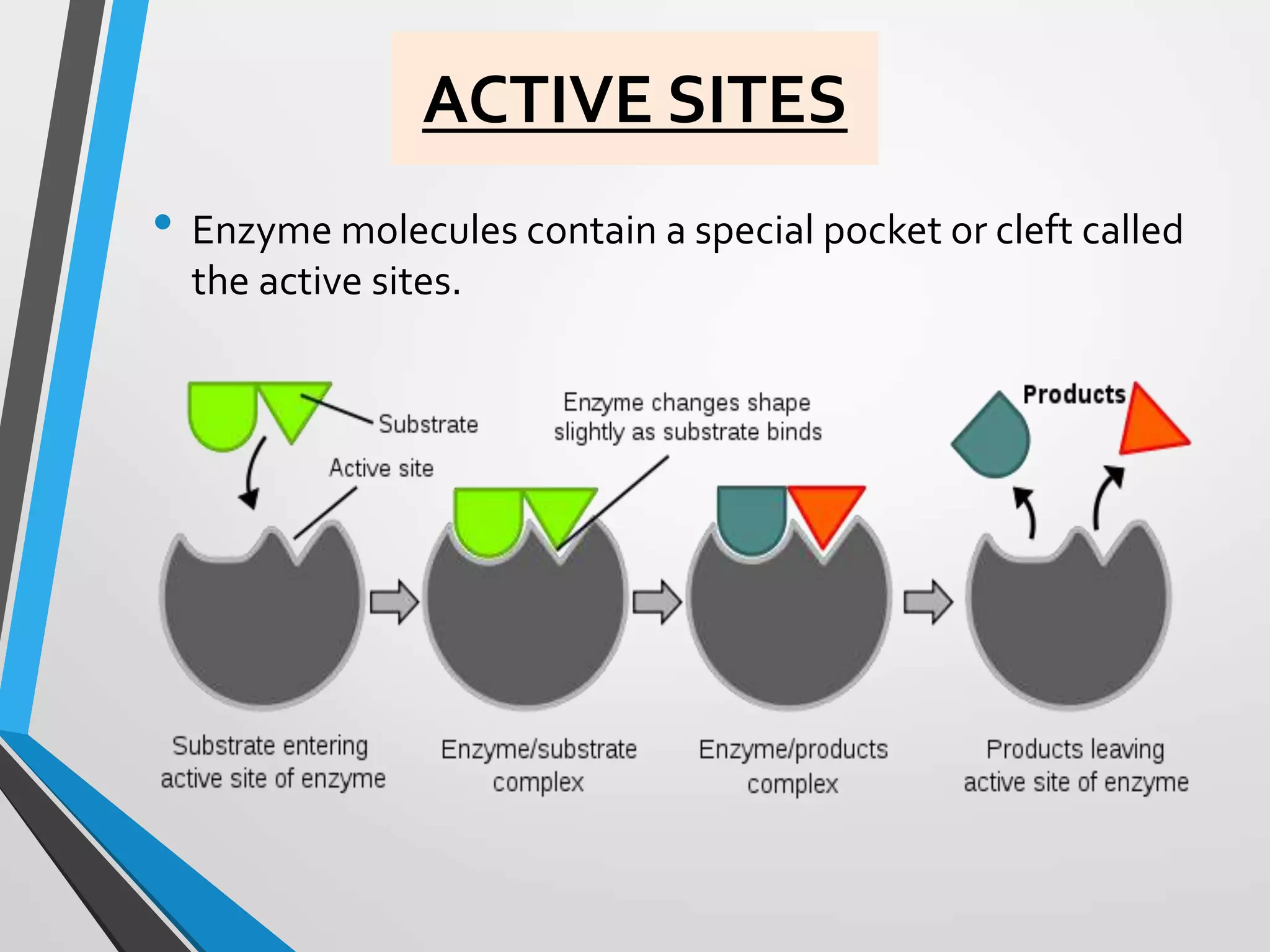
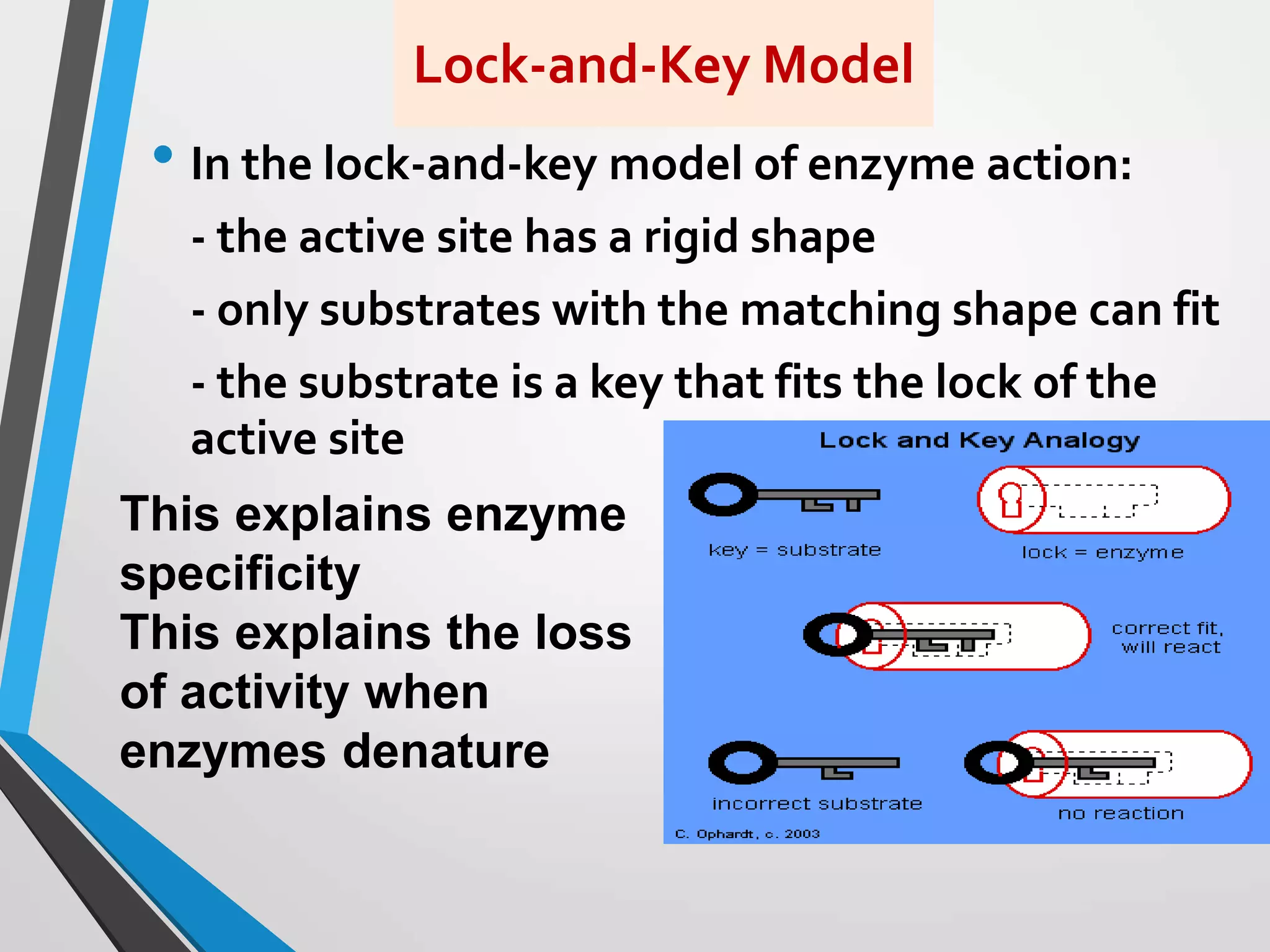
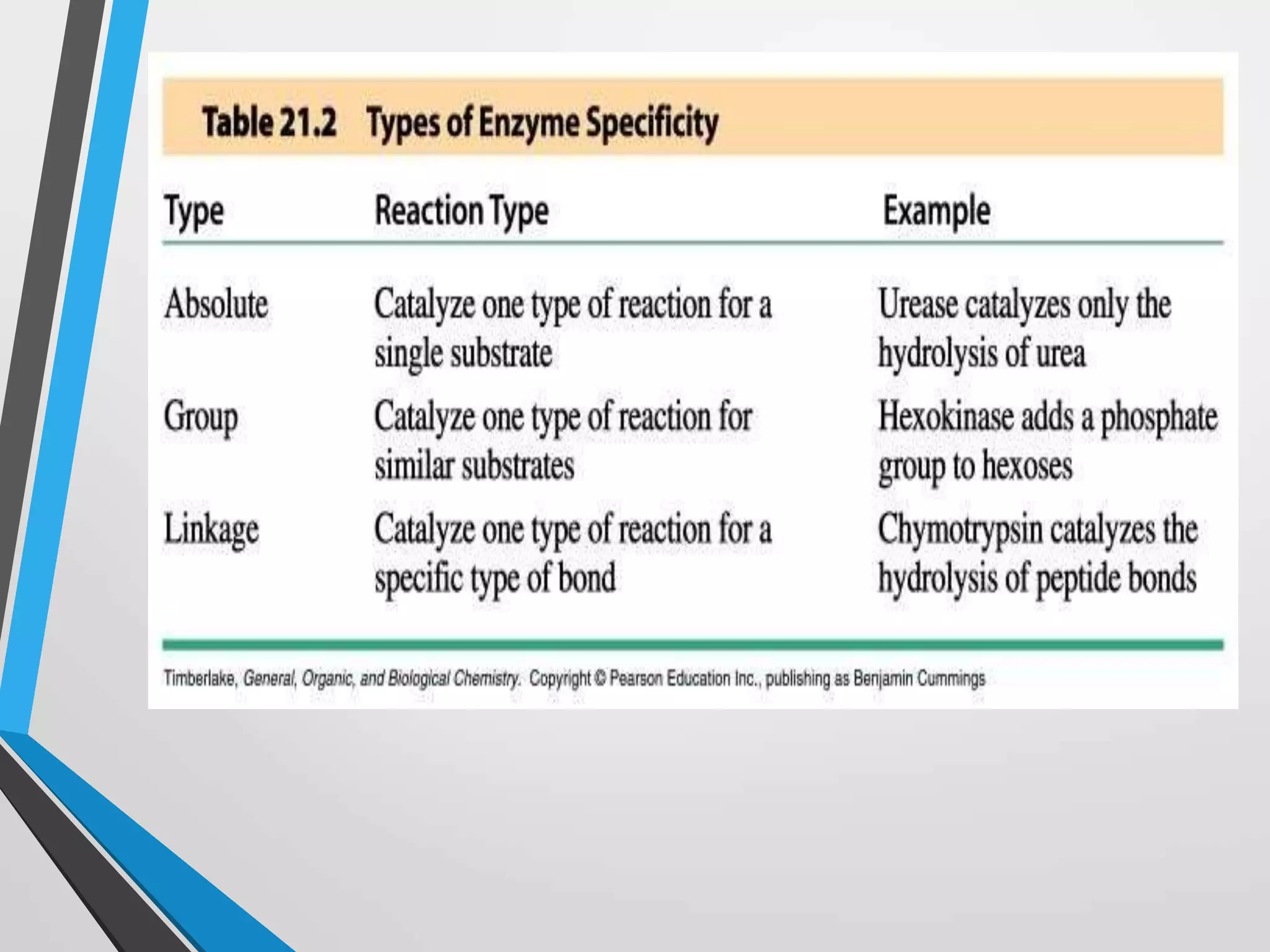
The document focuses on the concept of enzymes in pharmaceutical chemistry, defining key terms such as active site, apoenzyme, holoenzyme, and cofactor. It highlights the role of enzymes in metabolism, diagnosis, and therapeutics, emphasizing their function as biological catalysts that lower activation energy to facilitate biochemical reactions. Enzyme specificity and types of cofactors, including coenzymes and prosthetic groups, are also discussed.