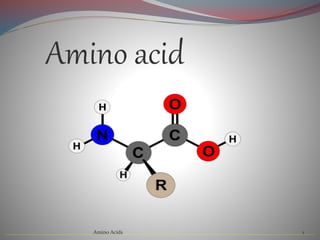
Amino acids are organic compounds that contain an amino group, a carboxyl group, a central carbon atom, and a specific side chain. They are classified as essential, non-essential, or conditional depending on whether the human body can synthesize them. Amino acids are the building blocks of proteins and participate in many important biological functions and cellular processes. They have various physical and chemical properties depending on their specific structure and side chain, and many amino acids have medicinal properties and play roles in treating diseases.