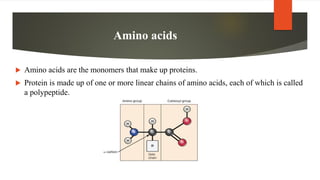
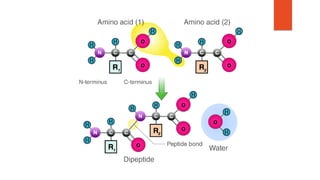
Proteins are made up of chains of amino acids that fold into complex 3D shapes defined by their sequence. There are 20 types of amino acids that can be combined to form the primary structure of a protein. The sequence determines the unique secondary, tertiary, and sometimes quaternary structures which define a protein's specific function. Proteins perform most of the work in cells and have roles in structure, function, regulation and catalysis. The seven main types of proteins include enzymes, structural proteins, transport proteins and more, each with distinct functions in the body.