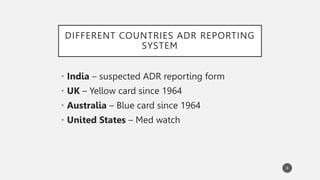
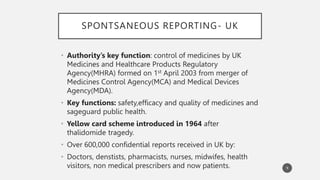
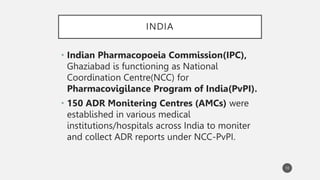
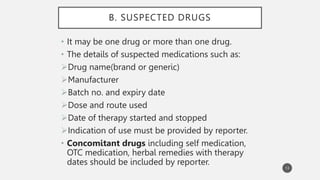
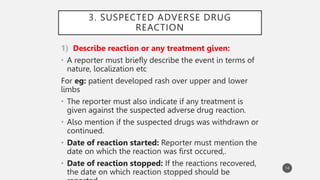
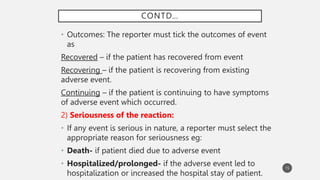
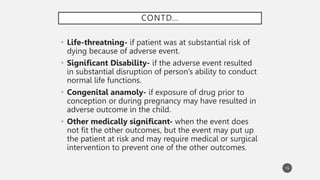
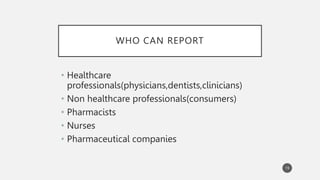
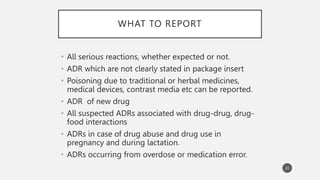
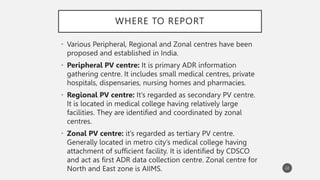
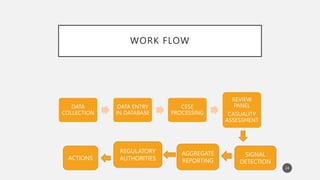
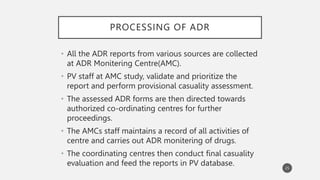
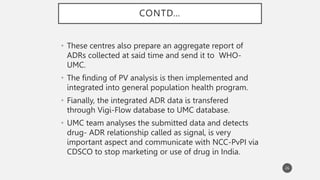
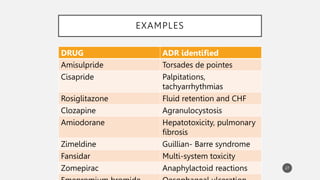
The document discusses spontaneous reporting systems and reporting adverse drug reactions (ADRs) to regulatory authorities. It describes the spontaneous reporting process which involves data acquisition from health professionals, data assessment of individual case reports and pooled data, and data interpretation to generate signals related to ADRs. Health professionals voluntarily report suspected ADRs directly to regulatory authorities or pharmaceutical companies. Various countries have different reporting systems, like the yellow card system in the UK. Underreporting remains a limitation of spontaneous reporting systems.













![REFERENCES
• “Guidanace Document for Spontnaeous Adverse Drug
Reaction Reporting”, Spontaneous Reporting,
Published by Indian Pharmacopoeia Commission,
NCC-PvPI, 2014, Page no: 10-17
• ‘Post marketing surveillance of suspected adverse
drug reactions through spontaneous reporting :
current status, challenges and future’ by Muaed
Alomar, Ali M Tawfiq, Nageeb Hassan and Subish
Palaian, Therapeutic Advances in Drug Safety,2020
[journals.sagepub.com]
• https://www.slideshare.net/ Spontaneous Reporting
by Sonal Pande.
31](https://image.slidesharecdn.com/spontaneousreportingsystemguidelinesforadrreporting-230726102412-7d7cb869/85/SPONTANEOUS-REPORTING-SYSTEM-GUIDELINES-FOR-ADR-REPORTING-pptx-31-320.jpg)