Embed presentation
Download to read offline


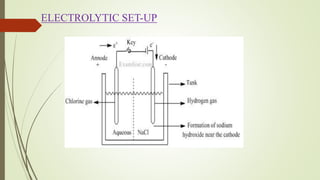




The chlor-alkali process uses electrolysis to produce chlorine, hydrogen, and sodium hydroxide from an aqueous sodium chloride solution. Electricity is passed through the solution, causing chloride ions to oxidize to chlorine gas at the anode and water molecules to reduce to hydrogen gas and hydroxide ions at the cathode. The sodium ions then react with the hydroxide ions to form sodium hydroxide. Chlorine is used to make polymers and pesticides, hydrogen is used as a reducing agent and fuel, and sodium hydroxide is used in soap, the paper industry, and water treatment.







