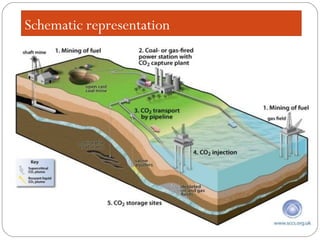
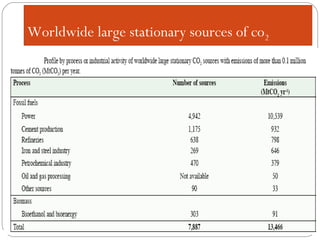
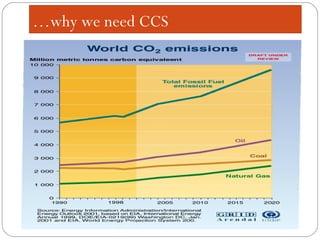
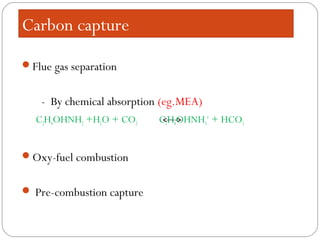
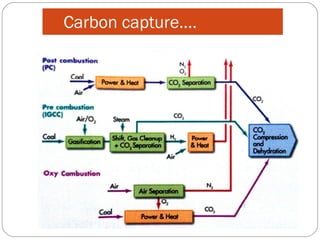
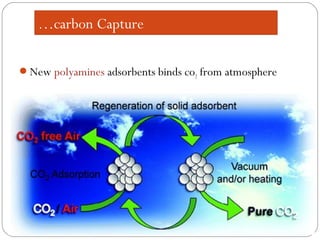
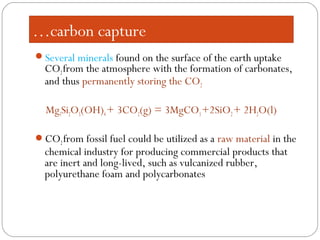
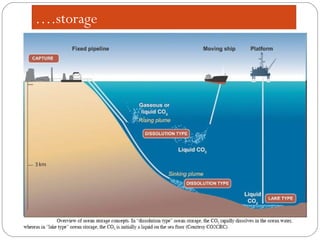
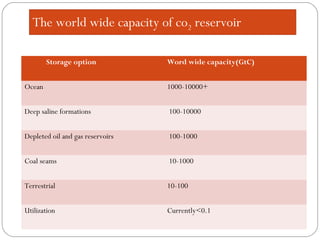
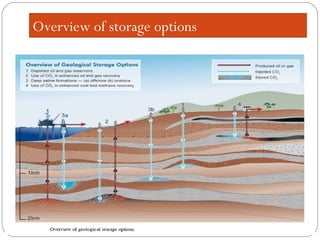
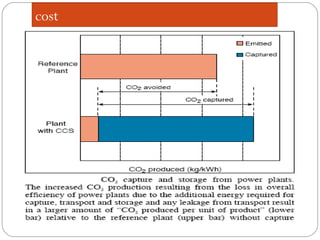
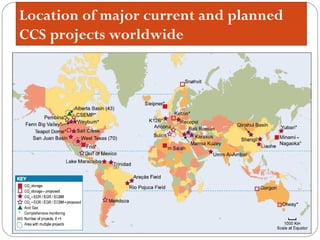
This document presents information on carbon capture and storage (CCS). It defines CCS as a process to separate CO2 from large industrial sources, transport it, and store it long-term to isolate it from the atmosphere. It discusses why CCS is needed to address rising CO2 levels and potential climate change. It also outlines the key components of CCS - carbon capture techniques, storage options like depleted oil/gas fields and saline aquifers, and costs. Finally, it briefly describes some existing CCS projects around the world.