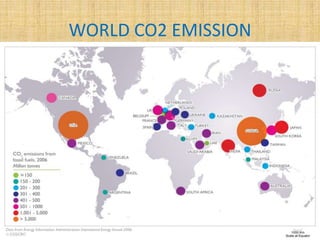
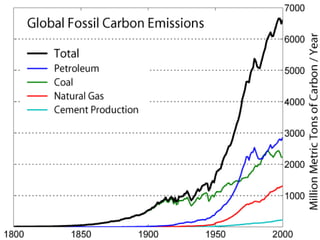
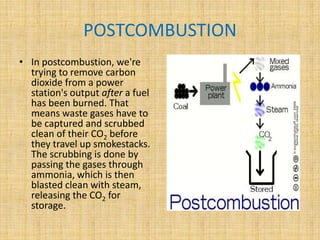
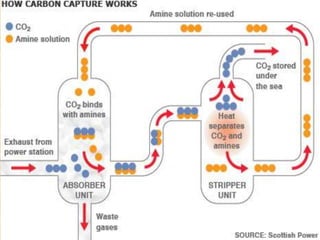
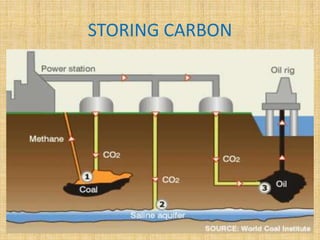
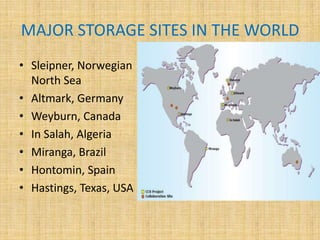
This document discusses carbon capture and storage (CCS) technologies which aim to prevent carbon dioxide emissions from fossil fuel use. It describes three main methods for capturing CO2 - pre-combustion, post-combustion, and oxyfuel combustion. The captured CO2 can be transported via pipeline and stored underground in geological formations or utilized for enhanced oil recovery. CCS has the potential to reduce CO2 emissions by 80-90% but also increases energy needs and costs for power plants. There are environmental concerns about the impacts of long-term CO2 storage or leakage.