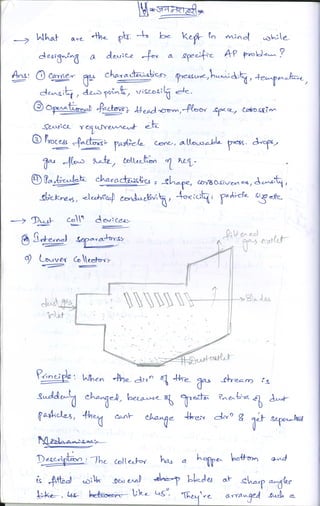
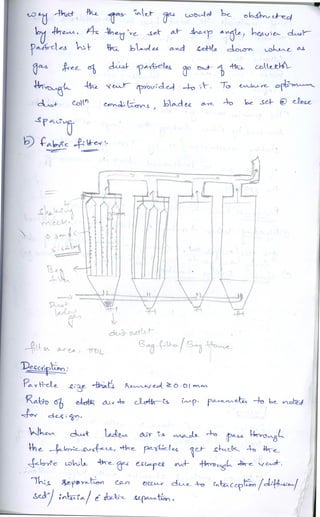
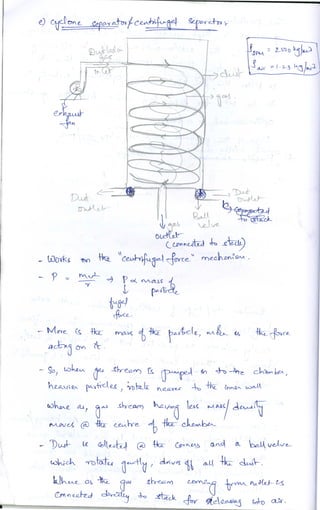
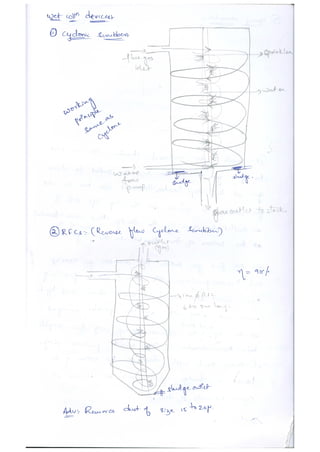
The document discusses different methods of removing particulate and gaseous pollutants from industrial emissions, including gravity settling chambers, cyclone separators, and absorption/adsorption techniques. Gravity settling relies on gravity for particle removal while cyclone separators utilize centrifugal force; both have pros and cons in terms of efficiency and design. Gaseous pollutants can be managed through physical and chemical methods such as absorption and adsorption, with absorption techniques involving both physical and chemical interactions between gases and liquids.