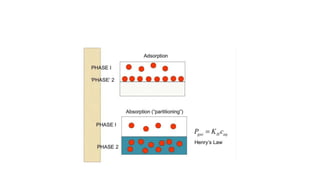
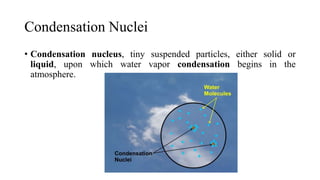
The document discusses various methods for controlling gaseous contaminants, including absorption, adsorption, condensation, and incineration. Absorption involves dissolving a gas in a liquid, such as water, through solubility and mass transfer. Adsorption accumulates molecules on surfaces through physical or chemical attraction. Condensation occurs when a gas changes to a liquid due to cooling and condensation nuclei. Incineration uses combustion and high temperatures to convert waste into ash, flue gas, and heat.