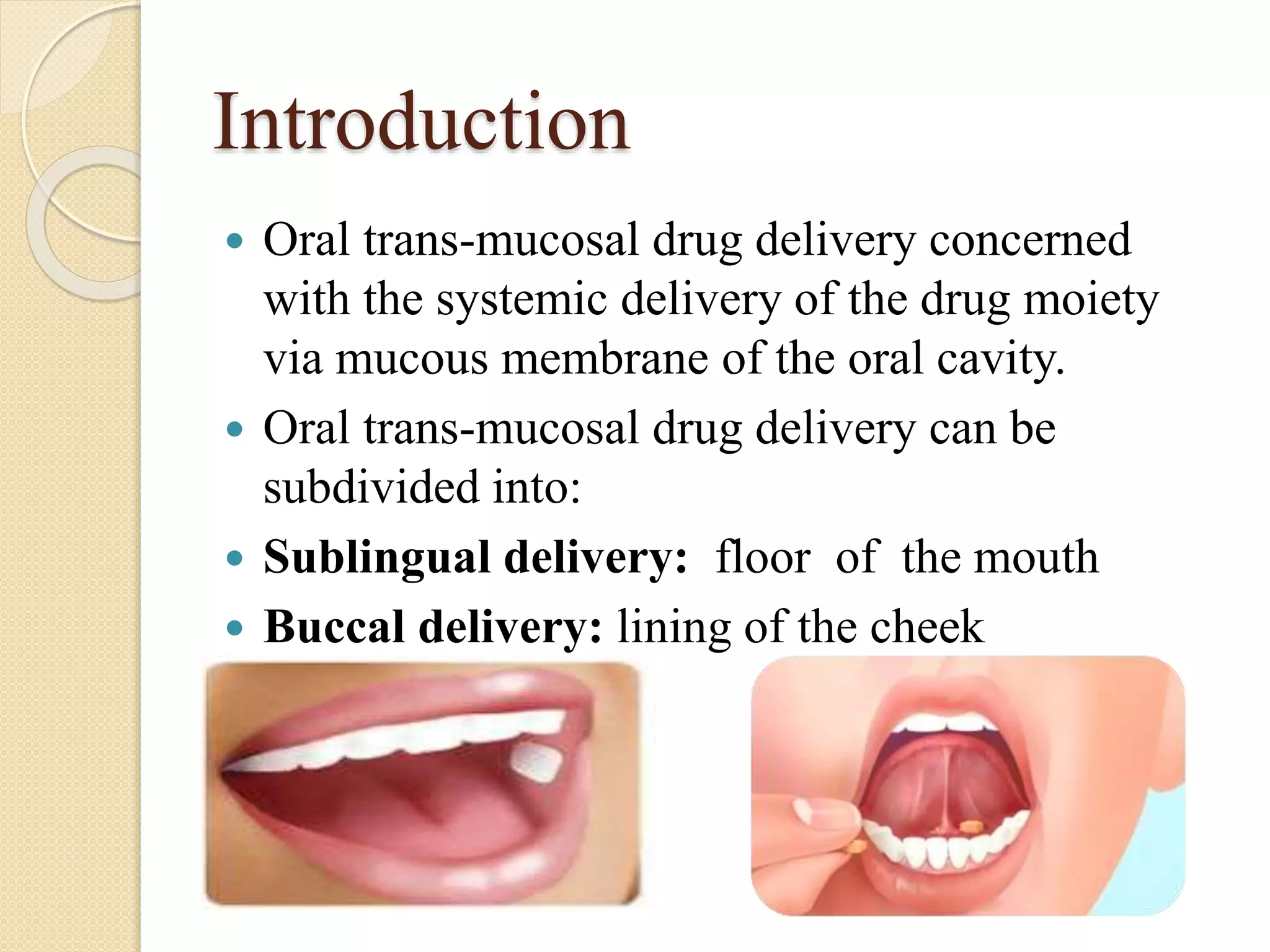
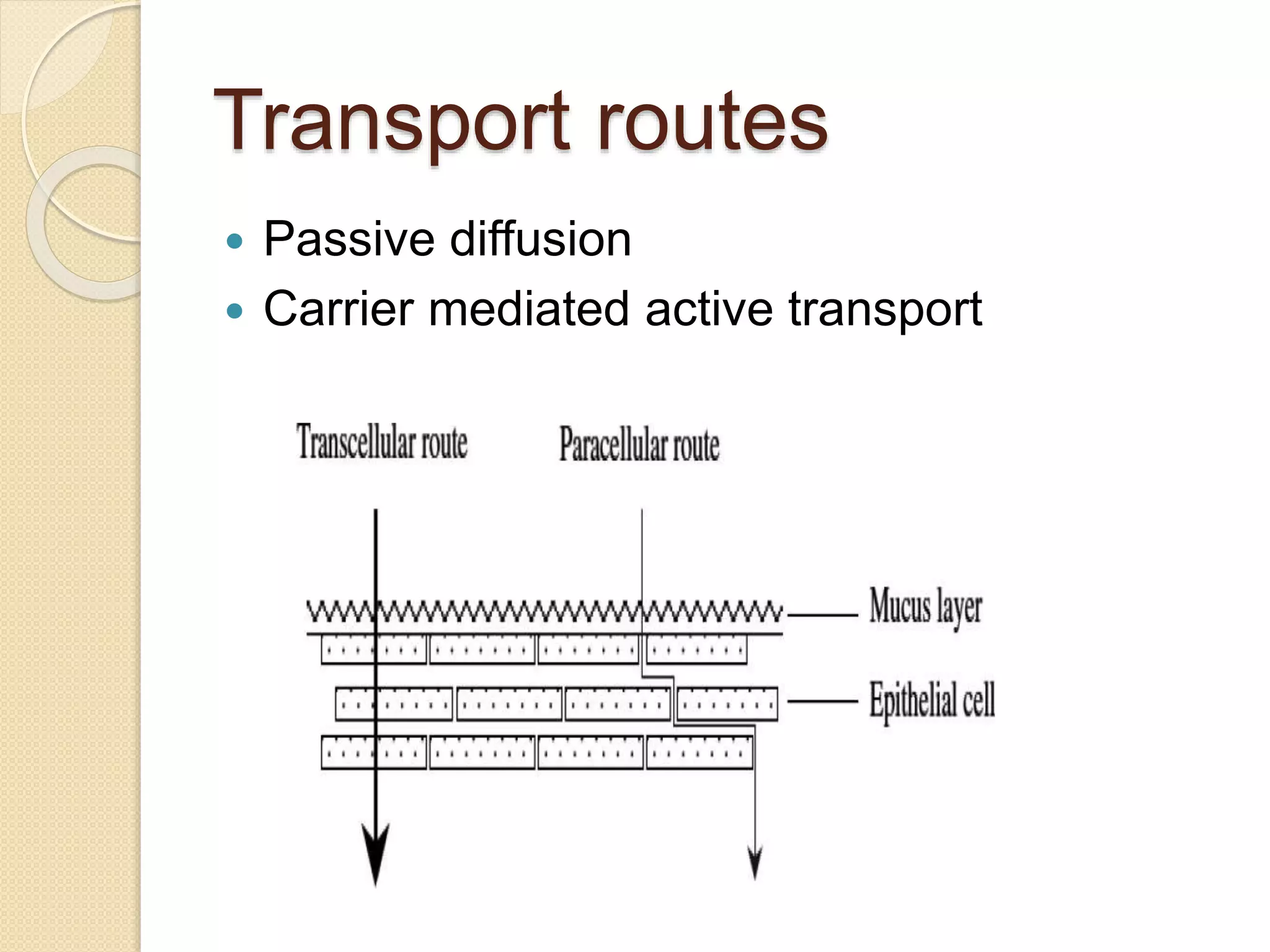
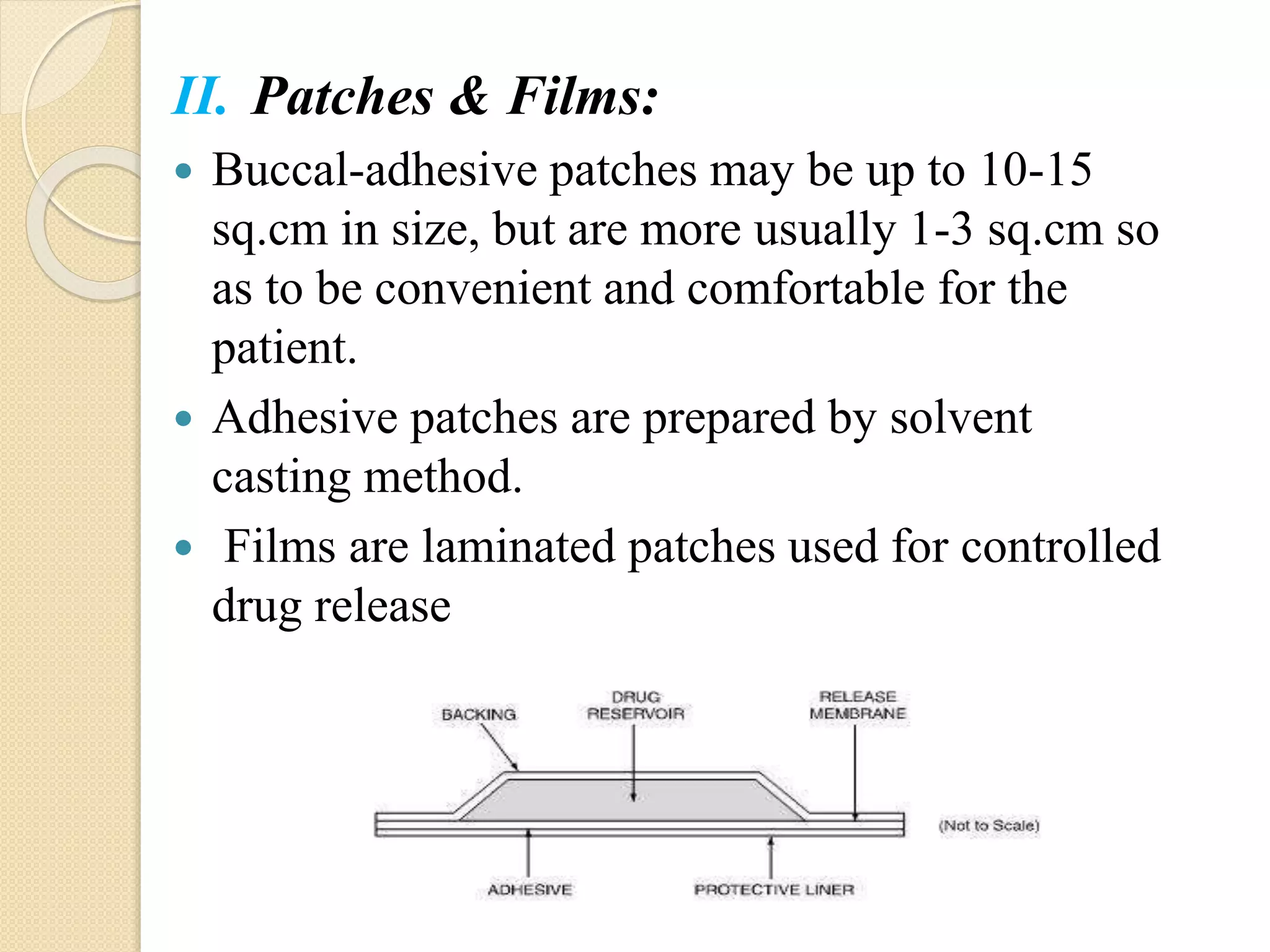
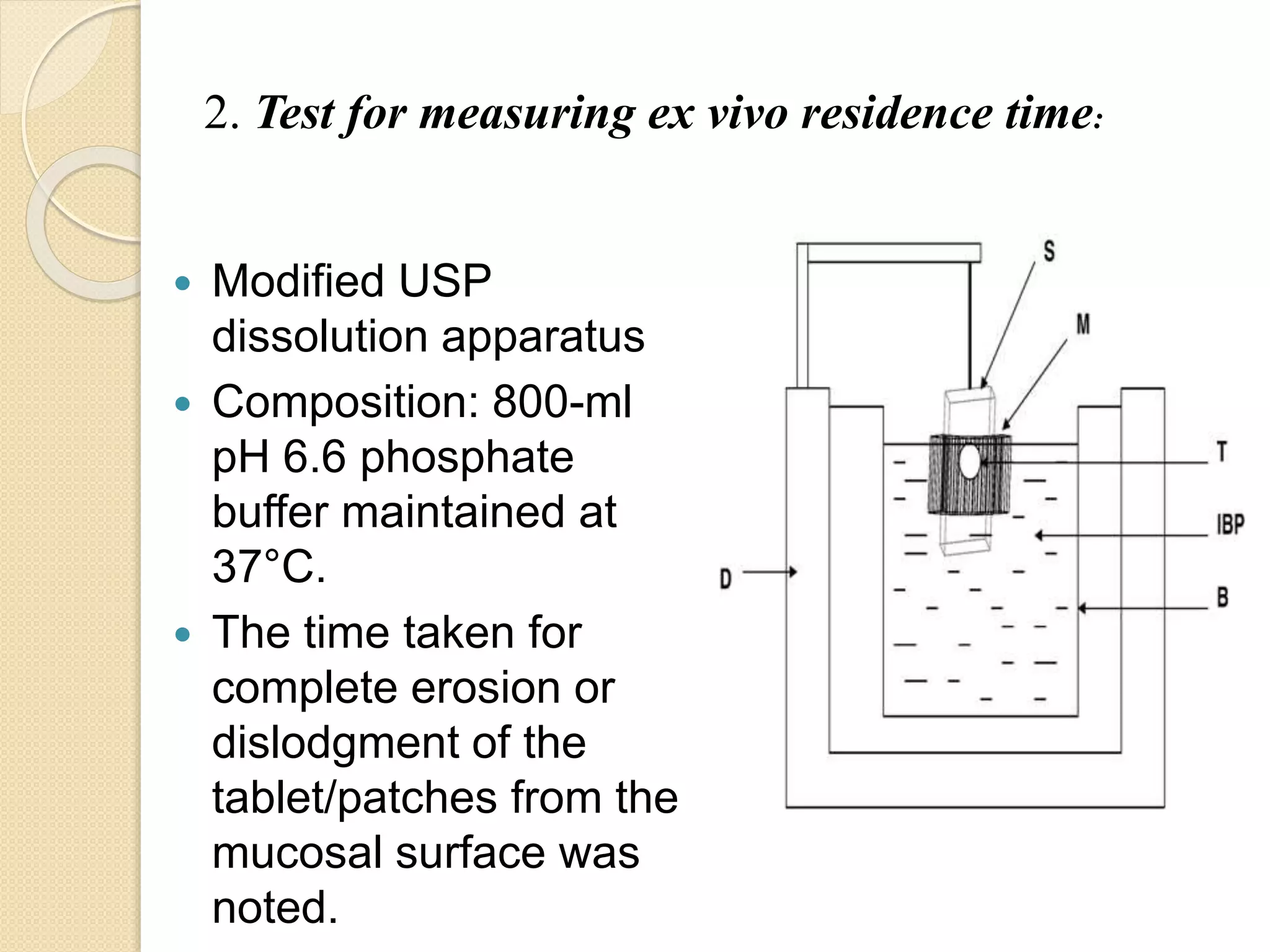
This document summarizes a presentation on advances in oral transmucosal drug delivery. It discusses how oral transmucosal delivery can avoid first-pass metabolism and includes sublingual and buccal delivery. It covers the anatomy of the oral mucosa, transport routes, theories of mucoadhesion, formulation considerations including basic components, and methods for evaluating formulations. The presentation provides an overview of oral transmucosal drug delivery and factors to consider for formulation and evaluation.