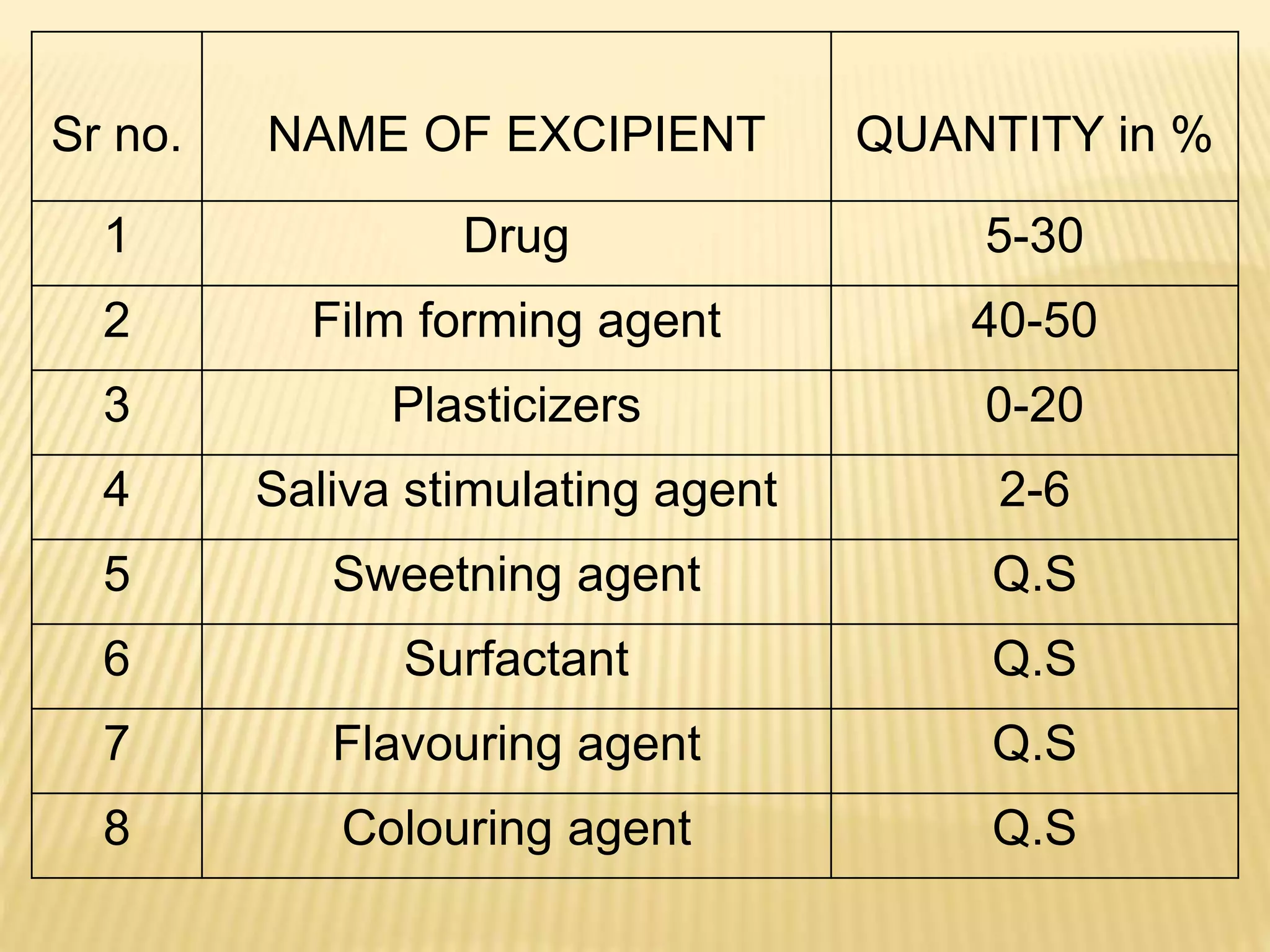
This document provides an overview of buccal films as a novel drug delivery system. It discusses the advantages of buccal administration such as rapid drug absorption and avoidance of first-pass metabolism. The document covers various aspects of buccal film formulation such as excipients, drug candidates, manufacturing methods, and evaluation tests. It presents examples of recent research on buccal films and concludes that this dosage form is well-suited for delivering drugs to patients across all age groups.