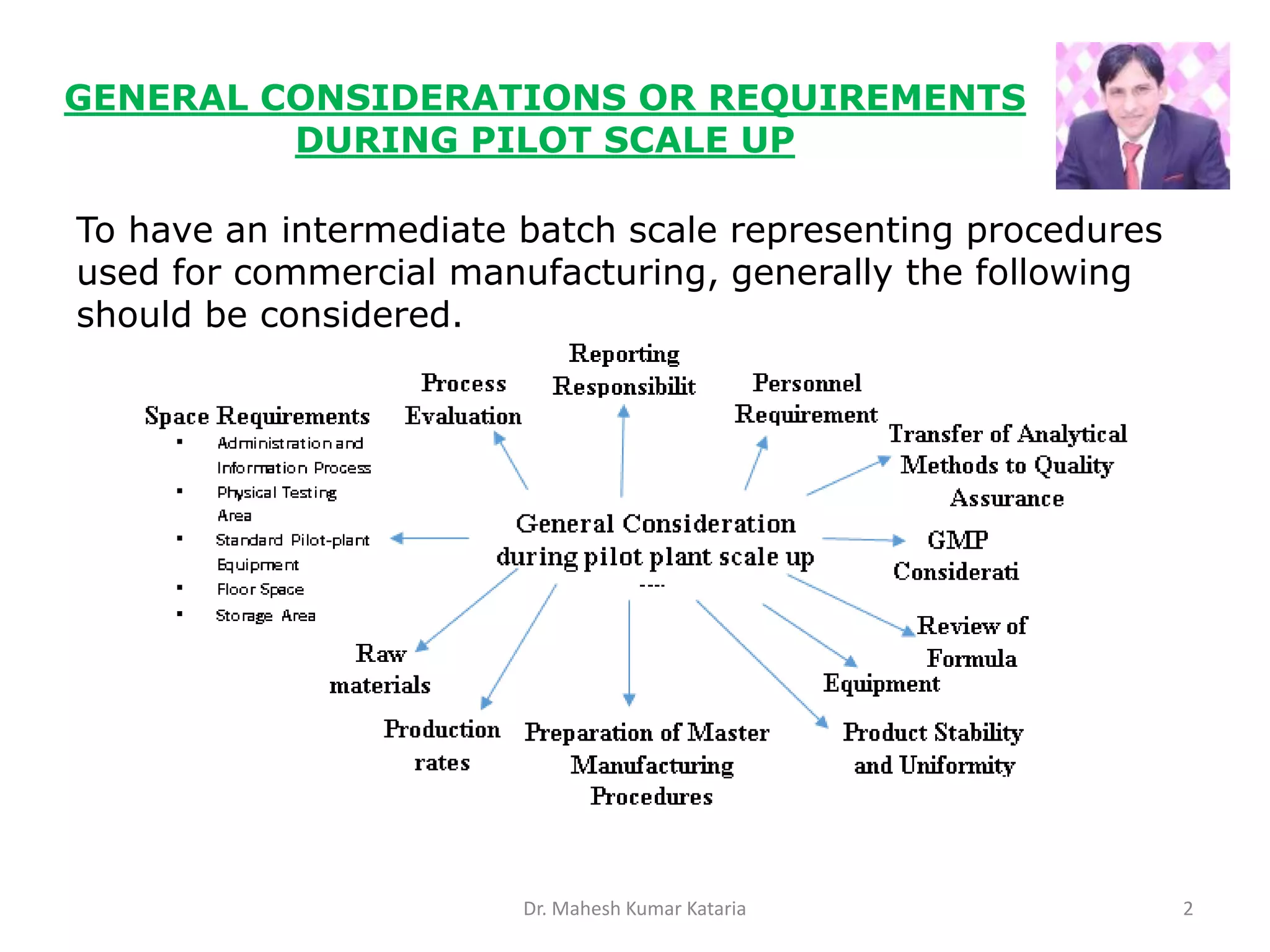
This document discusses general considerations for pilot plant scale up techniques. It outlines 12 key areas that should be considered when scaling up a formulation from the laboratory to a pilot plant scale, including reporting responsibilities, personnel requirements, space requirements, reviewing the formula, raw materials, processing equipment, production rates, process evaluation, manufacturing procedures, product stability and uniformity, GMP compliance, and transferring analytical methods to quality assurance. The goal is to produce a formulation on an intermediate batch scale that represents the procedures used for commercial manufacturing.