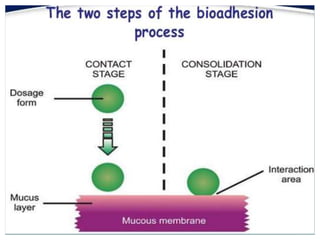
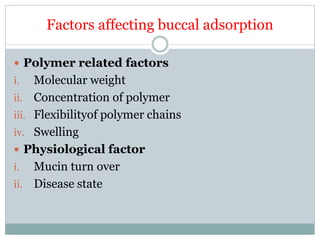
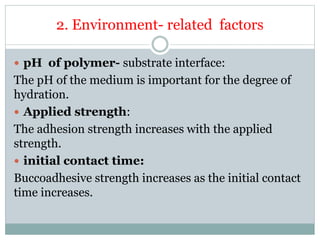
The document discusses buccal drug delivery systems (BDDS) which allow for drug administration through the buccal mucosa, providing advantages such as avoidance of first-pass metabolism and ease of administration. It covers aspects such as the structure and physiology of oral mucosa, the formulation of BDDS, mechanisms of mucoadhesion, and factors affecting drug absorption. Evaluative techniques for buccal patches are also outlined, emphasizing the importance of polymer characteristics and environmental factors in enhancing drug delivery efficacy.































![ 3. Swelling study: Buccal patches are weighed
individually (designated as W1), and placed separately in
2% agar gel plates, incubated at 37°C ± 1°C, and examined
for any physical changes. At regular 1- hr time intervals
until 3 hours, patches are removed from the gel plates and
excess surface water is removed carefully using the filter
paper[46] . The swollen patches are then reweighed (W2)
and the swelling index (SI) is calculated using the following
formula.
SI=W2-W1 × 100
W1](https://image.slidesharecdn.com/buccaldrugdeliverysystemfinal-180112084230/85/Buccal-drug-delivery-system-39-320.jpg)






