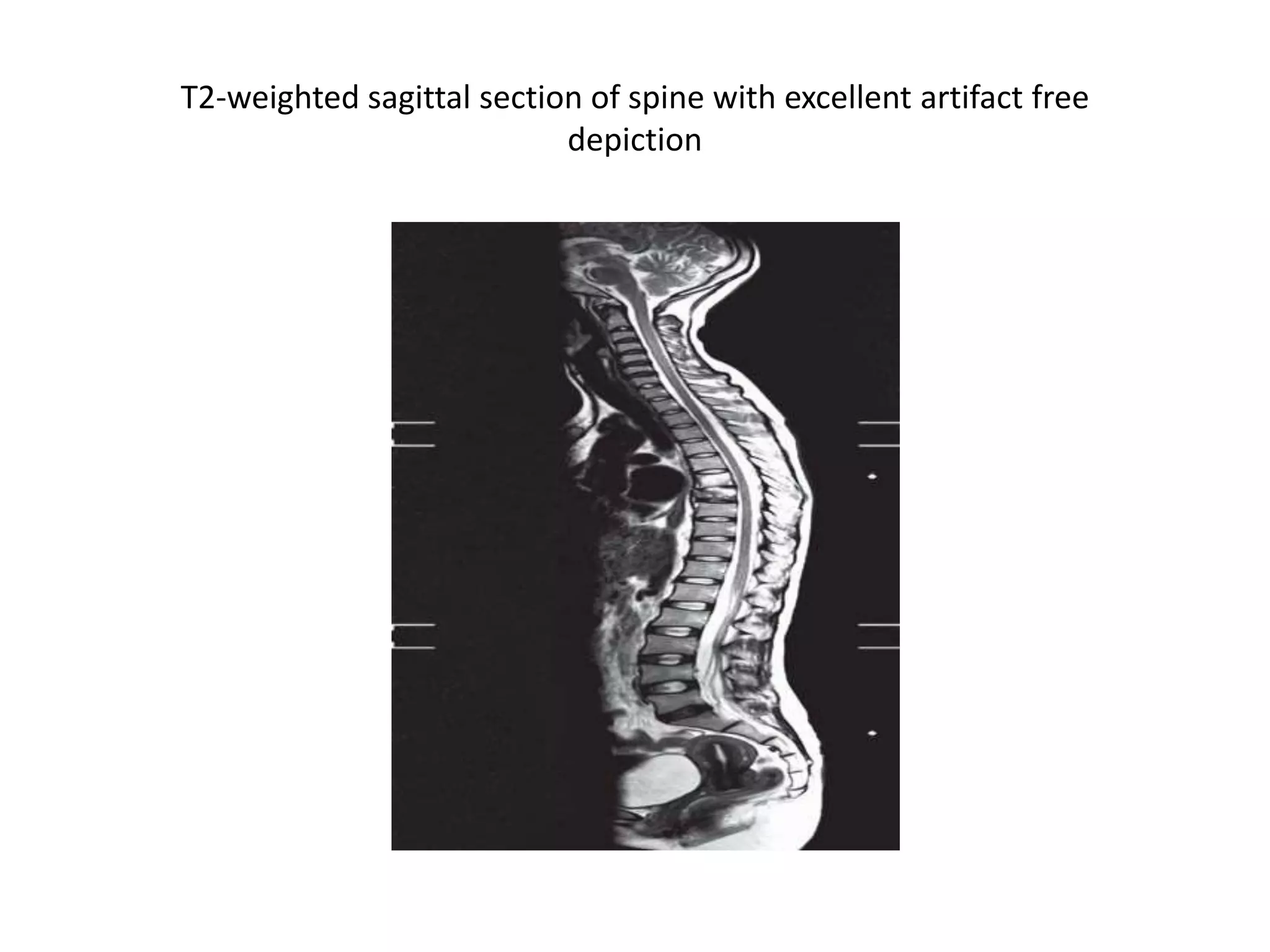
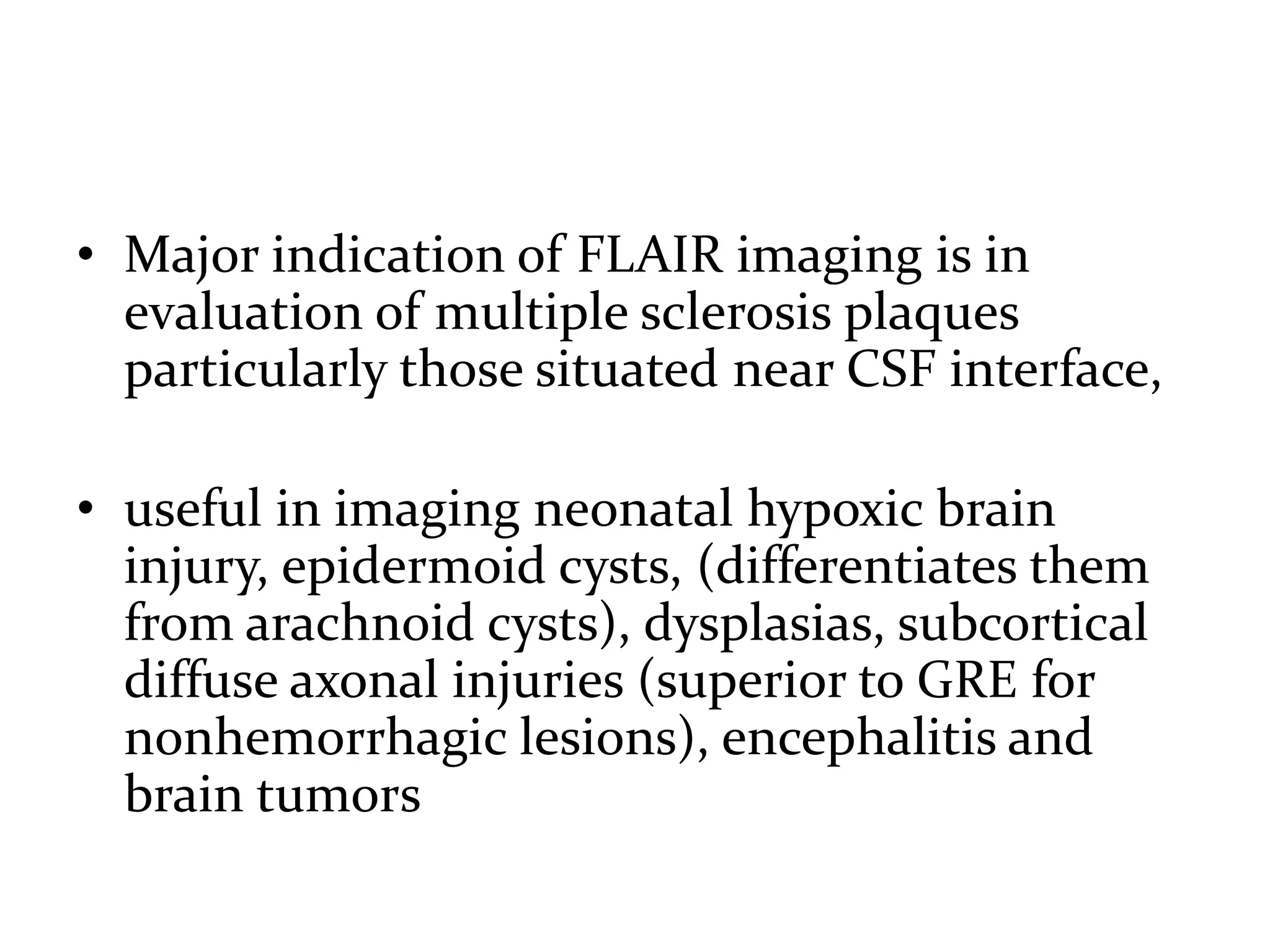
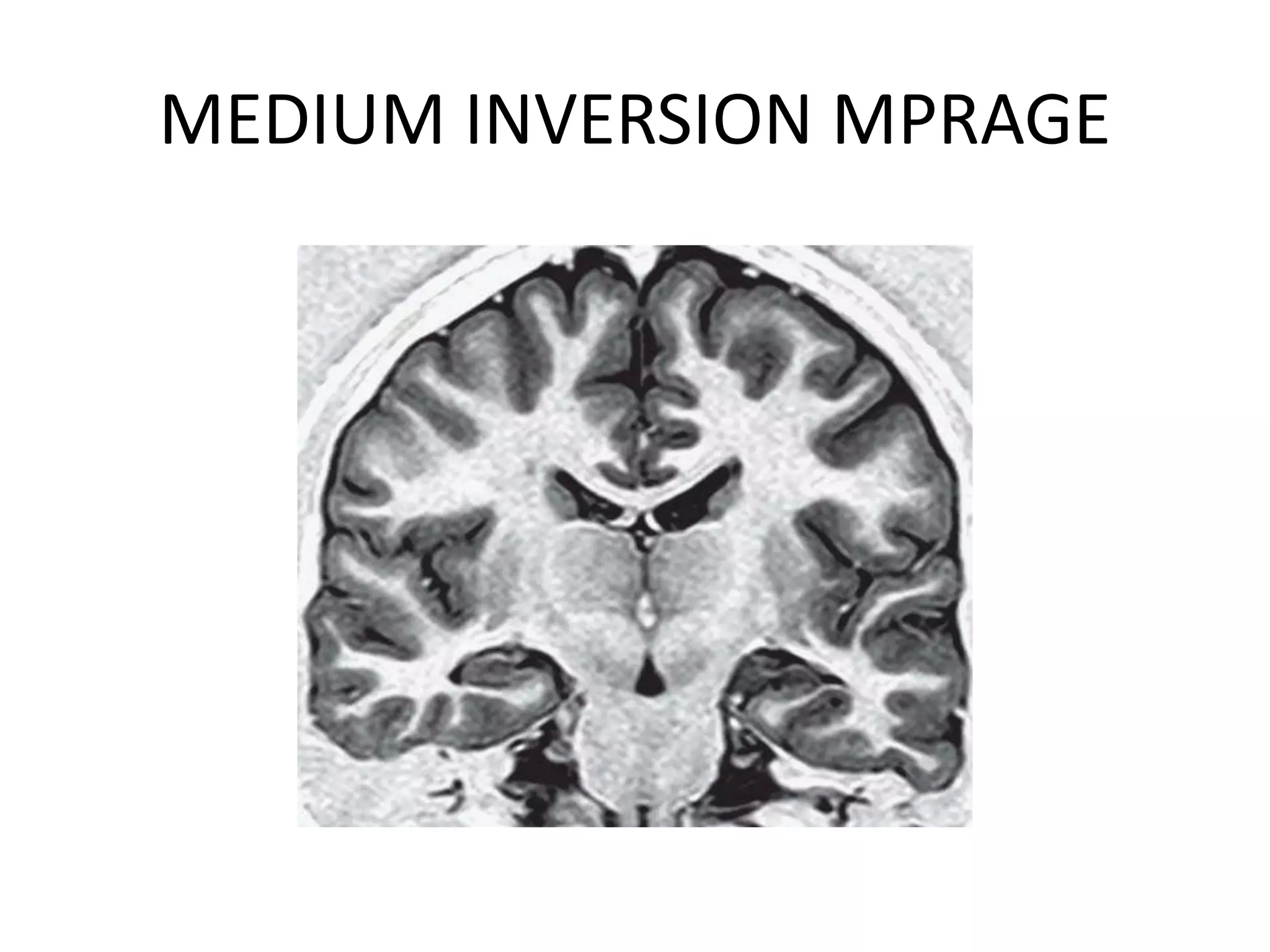
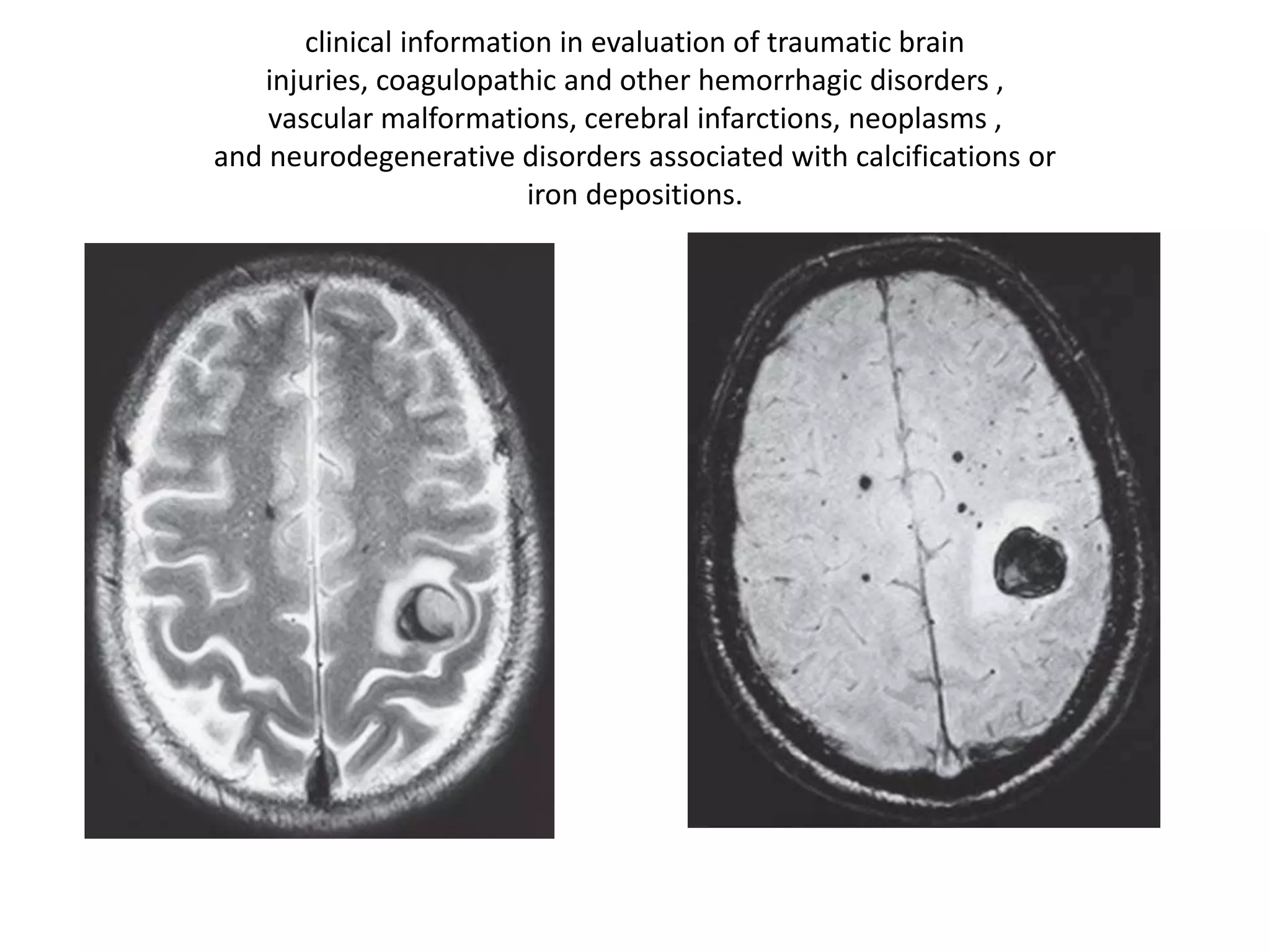
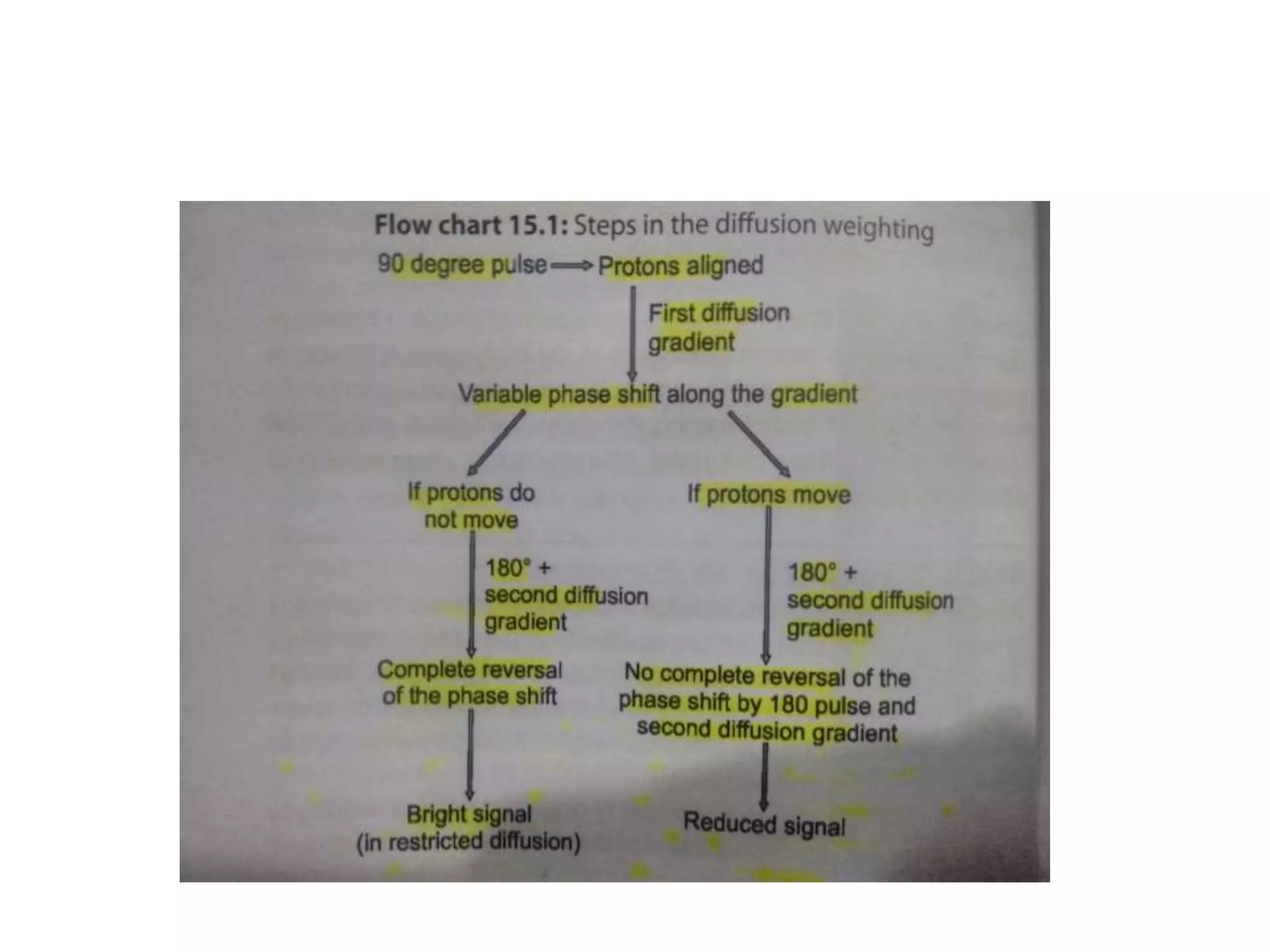
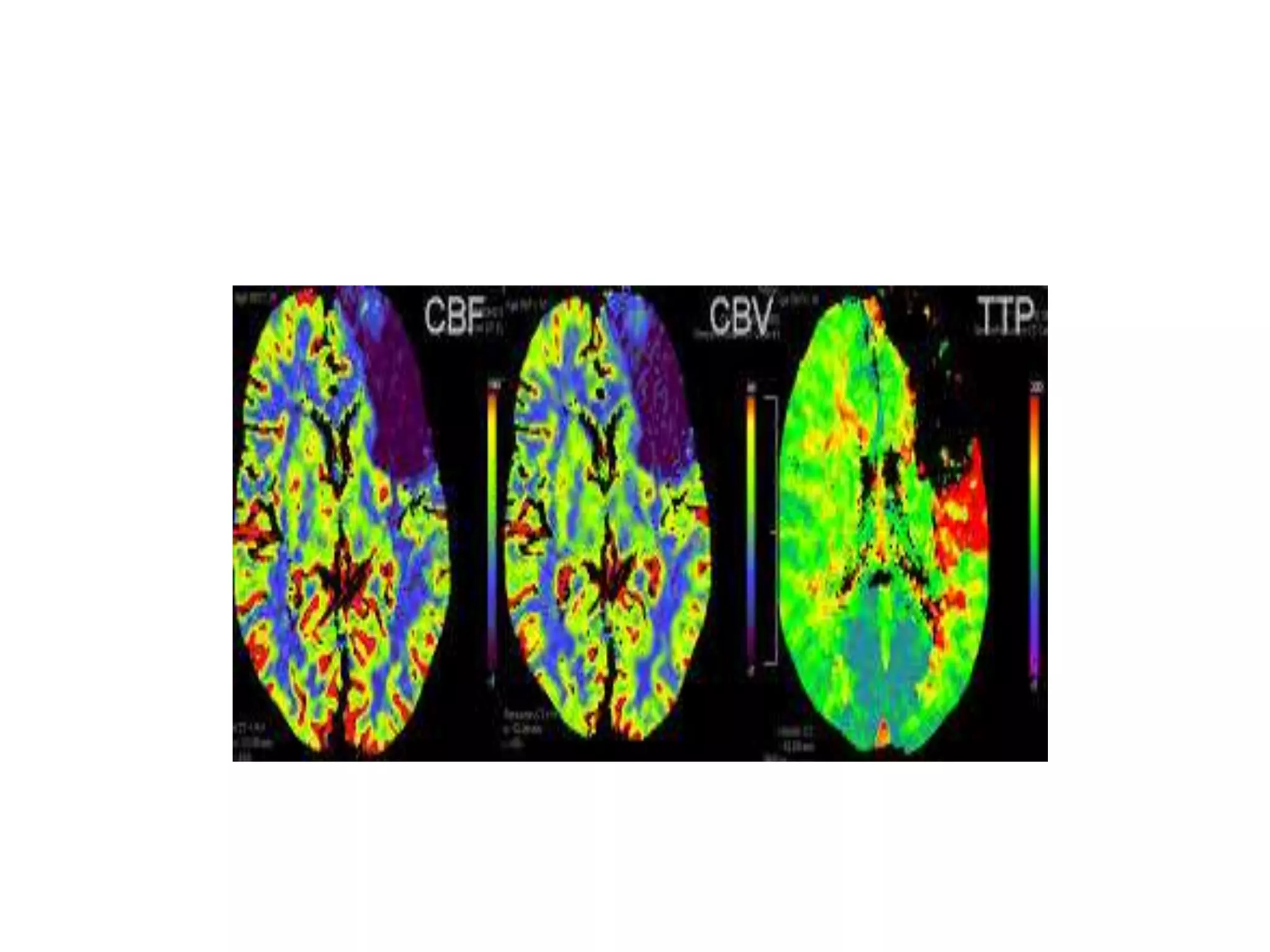
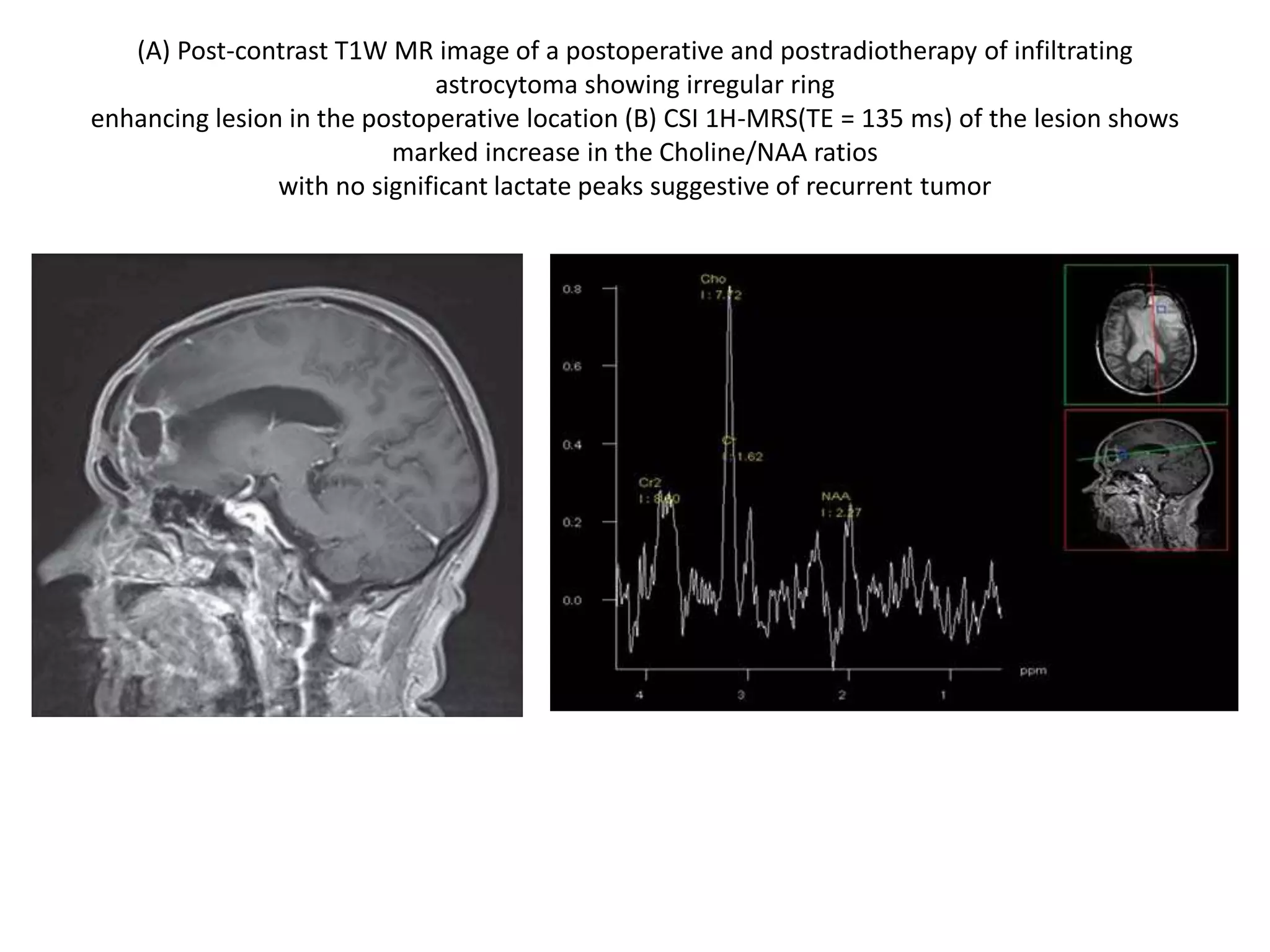
This document discusses advances in neuroimaging techniques using MRI. It covers 4 basic steps in MRI including placing the patient in a magnet, sending and receiving radiofrequency pulses, and transforming signals into images. Improvements discussed include large field of view imaging using sliding tables, high field strength 3T imaging for improved resolution, efficient data processing techniques like parallel imaging, and improved pulse sequences. Specific sequences covered are fast spin echo, fluid attenuated inversion recovery, gradient echo imaging, steady state sequences, susceptibility weighted imaging, diffusion imaging, perfusion imaging, and magnetic resonance spectroscopy.