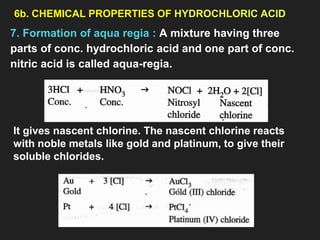
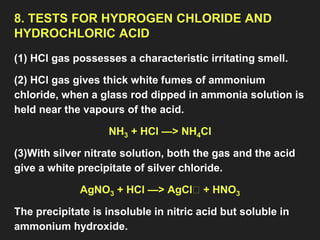
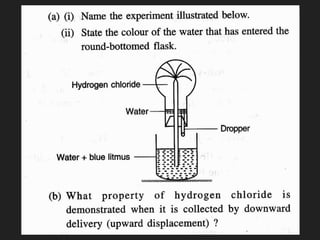
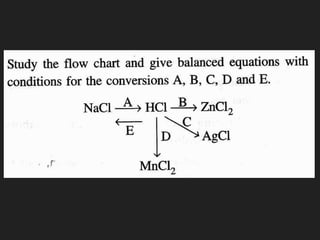
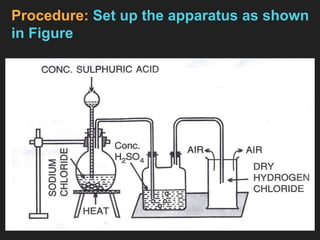
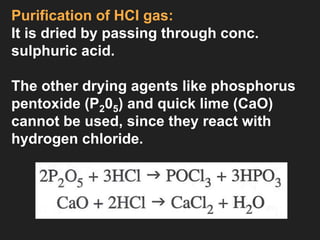
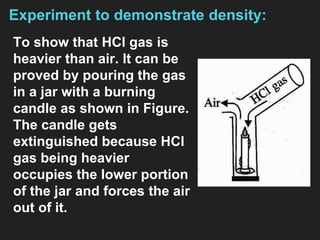
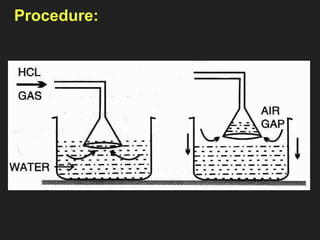
This document provides information about the study of the compound hydrogen chloride. It discusses the preparation of hydrogen chloride gas from sodium chloride, including the laboratory method and reaction. It also describes experiments to show the density and solubility of hydrogen chloride gas. The preparation of hydrochloric acid by dissolving hydrogen chloride gas in water is explained. The physical and chemical properties of both hydrogen chloride gas and hydrochloric acid are outlined. Uses of hydrochloric acid include industrial applications and as a medicine. Tests for identifying hydrogen chloride gas and hydrochloric acid involve reactions with ammonia and silver nitrate that produce precipitates.
![Study of Compounds A:
Hydrogen Chloride
Grade - 10 [ICSE]](https://image.slidesharecdn.com/ahydrogenchloride-231225161145-1225ed23/85/_A_-Hydrogen-Chloride-pptx-1-320.jpg)


![Learning Objectives
1. Introduction
2. Occurrence
3. Preparation of Hydrogen Chloride Gas
4. Properties of HCl Gas [Physical & Chemical]
5. Preparation of Hydrochloric acid
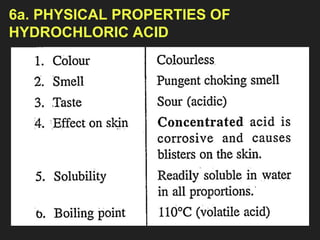
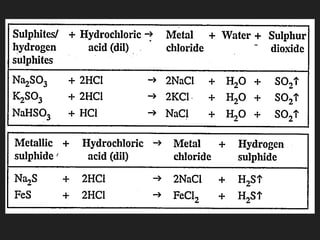
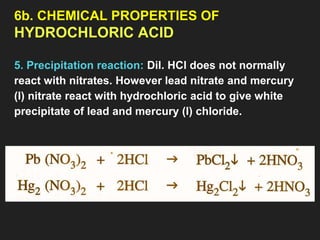
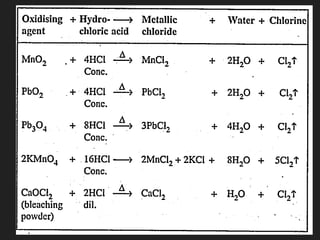
6. Properties of HCl acid [Physical & Chemical]
7. Uses
8. Tests for HCl gas and HCl acid](https://image.slidesharecdn.com/ahydrogenchloride-231225161145-1225ed23/85/_A_-Hydrogen-Chloride-pptx-4-320.jpg)

















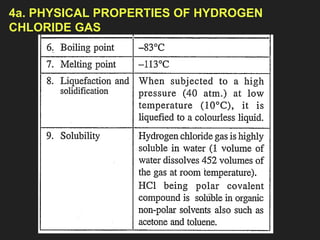
![4. Properties of HCl Gas
[Physical & Chemical]](https://image.slidesharecdn.com/ahydrogenchloride-231225161145-1225ed23/85/_A_-Hydrogen-Chloride-pptx-24-320.jpg)

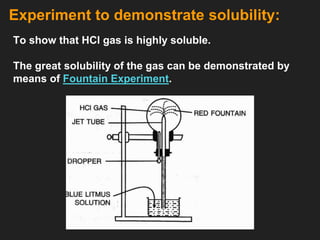

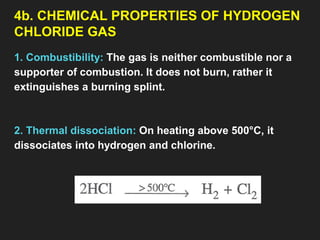
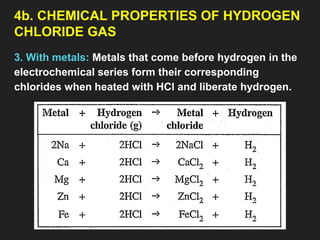


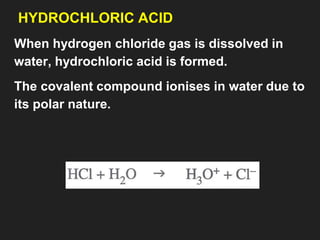








![6. Properties of
Hydrochloric acid
[Physical & Chemical]](https://image.slidesharecdn.com/ahydrogenchloride-231225161145-1225ed23/85/_A_-Hydrogen-Chloride-pptx-44-320.jpg)