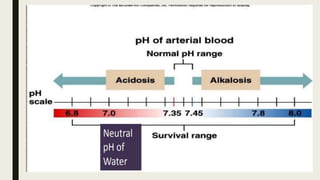
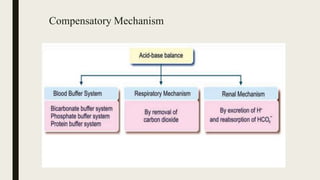
1. Acid-base balance is important for homeostasis and physiological functions. Acids are produced but balanced by bases to maintain pH.
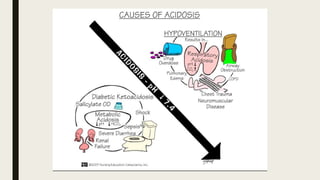
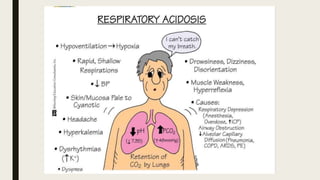
2. Respiratory acidosis occurs when carbon dioxide retention increases blood CO2 and decreases pH. Causes include hypoventilation. Treatment includes oxygen, identifying causes, and mechanical ventilation if needed.
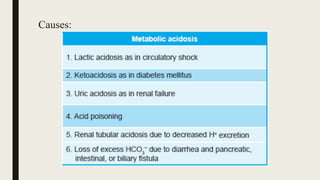
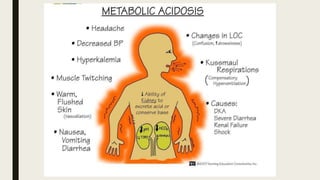
3. Metabolic acidosis results from excess organic acids from conditions like ketoacidosis, renal failure, or lactic acidosis. Treatment focuses on the underlying condition, fluid/electrolyte balance, and sodium bicarbonate or dialysis for severe cases.