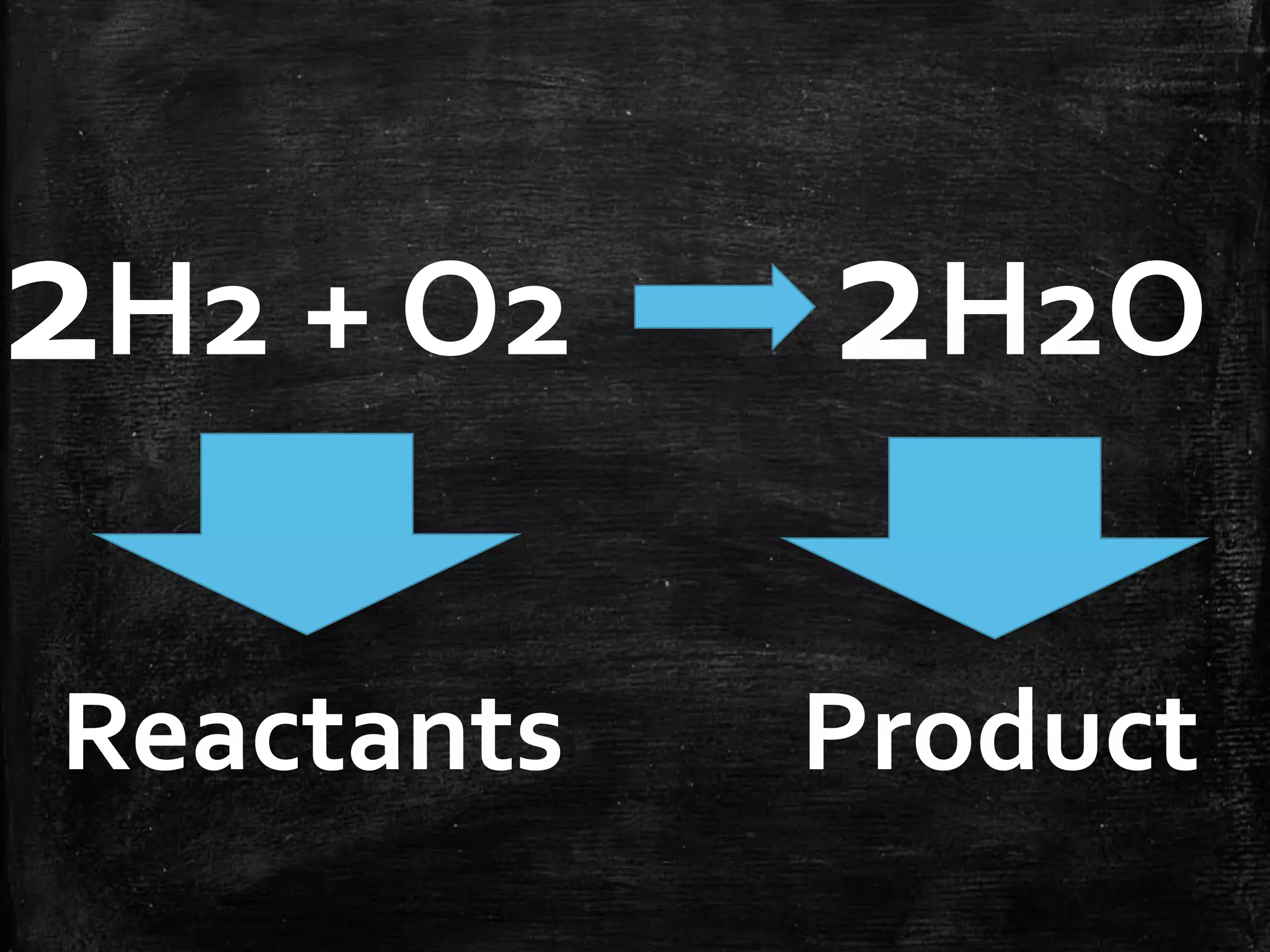
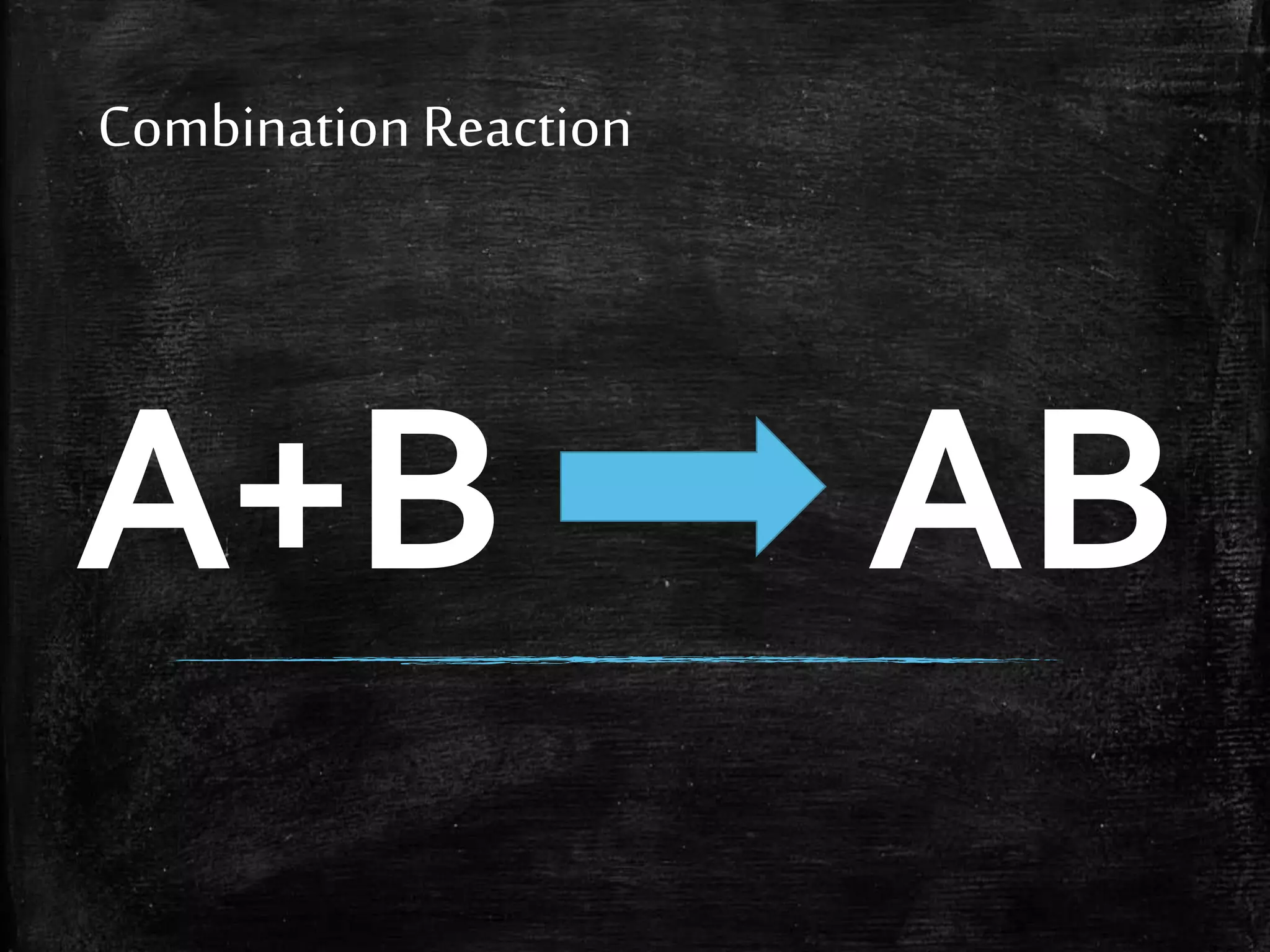
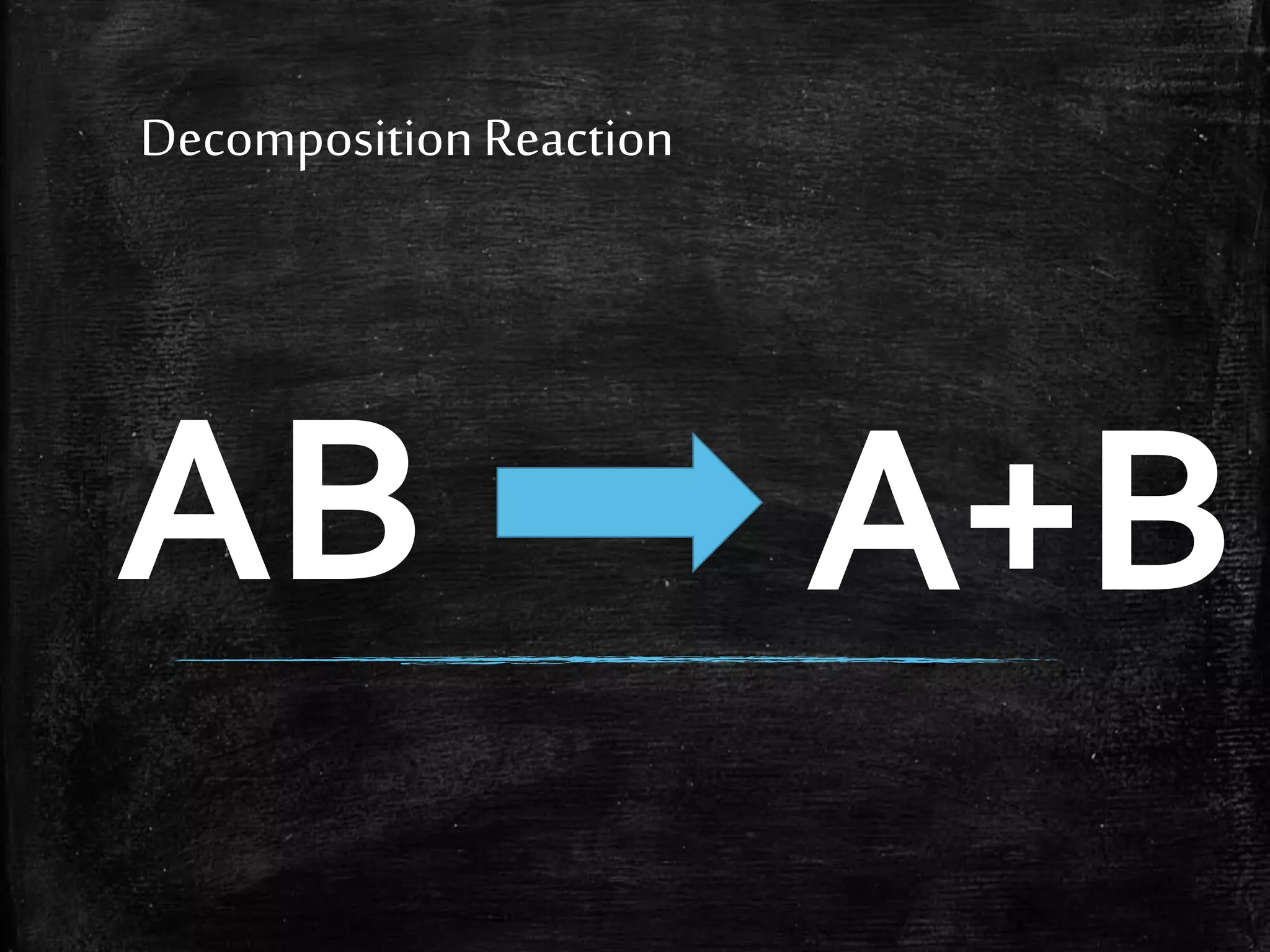
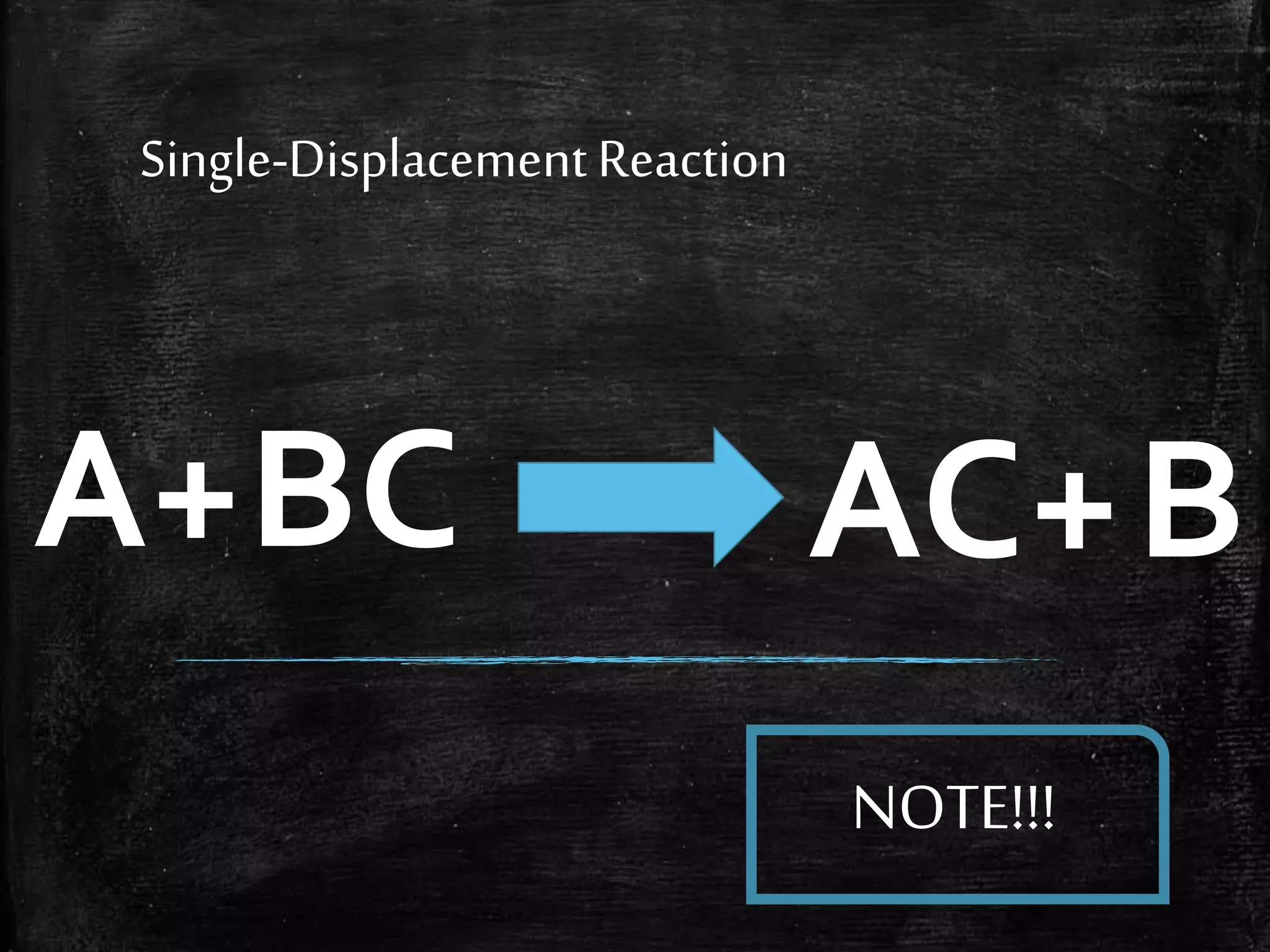
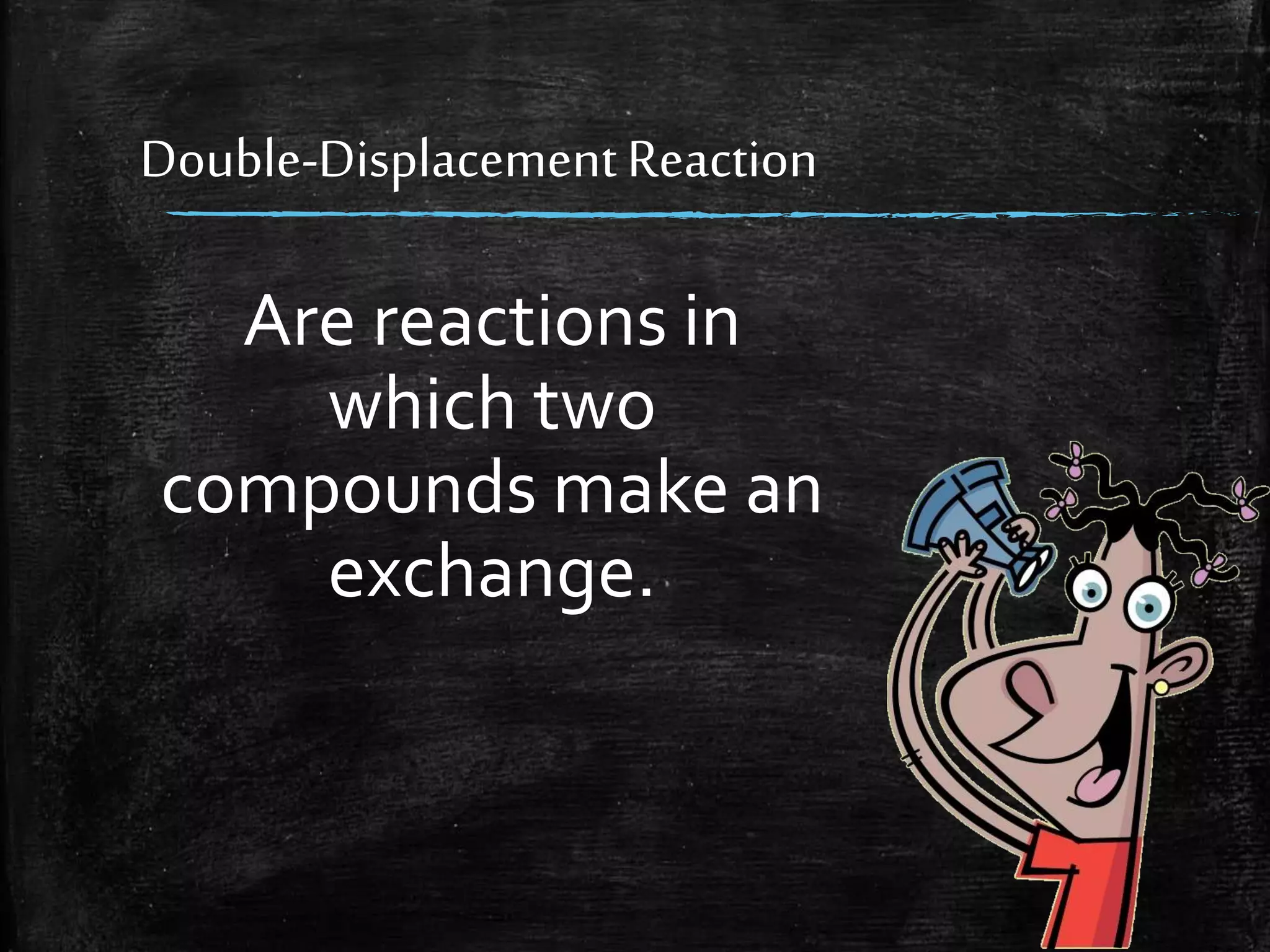
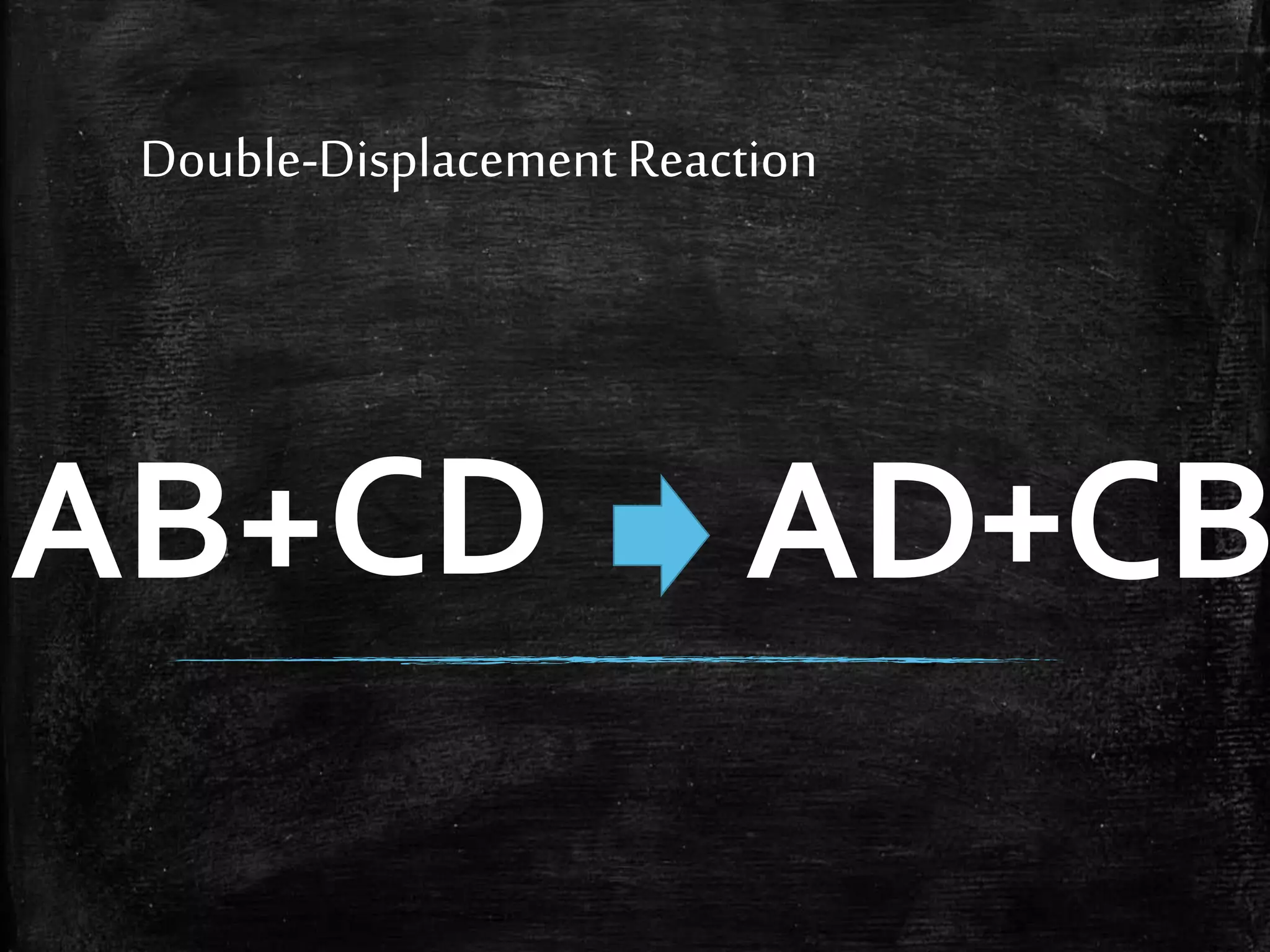
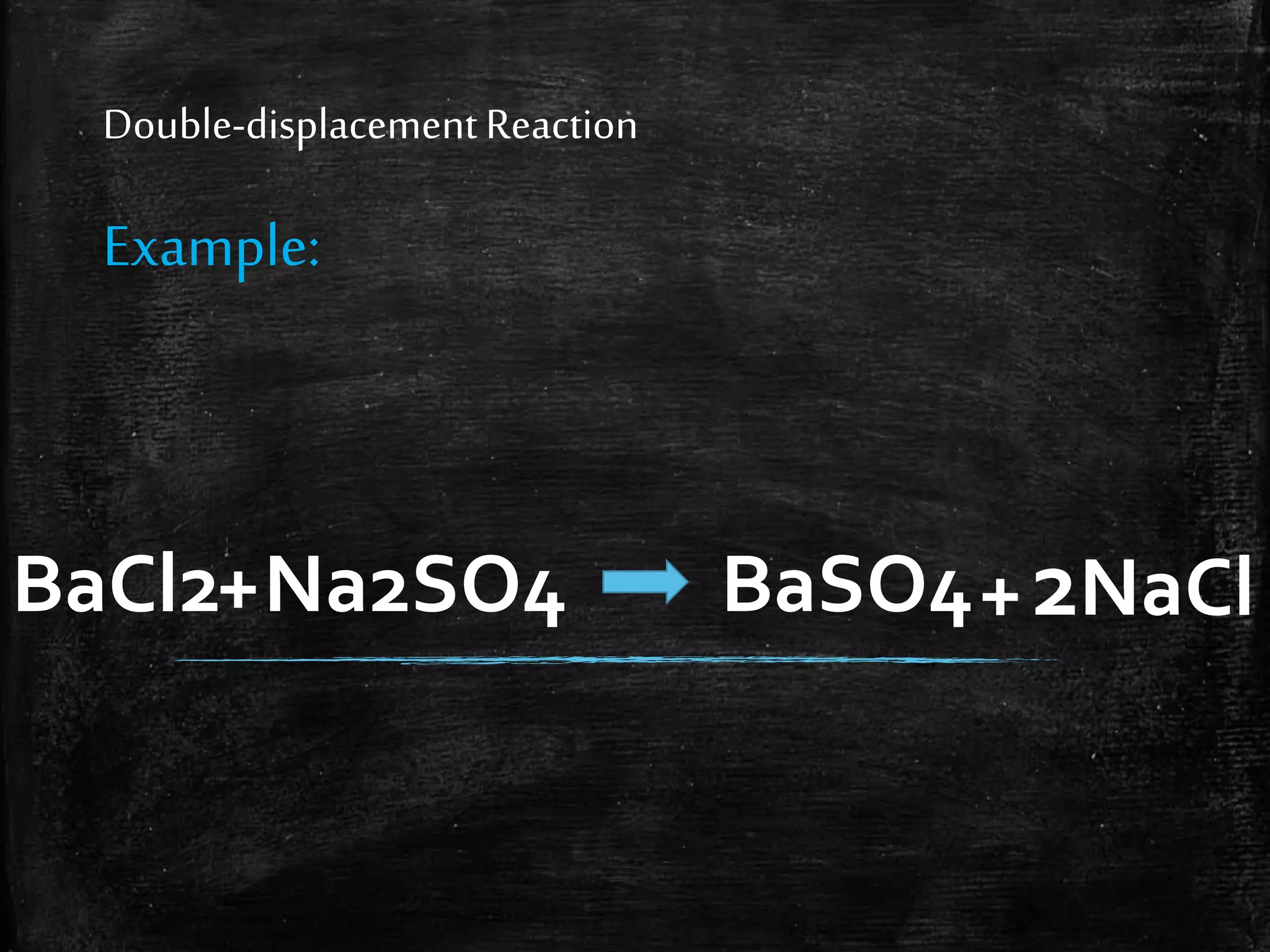
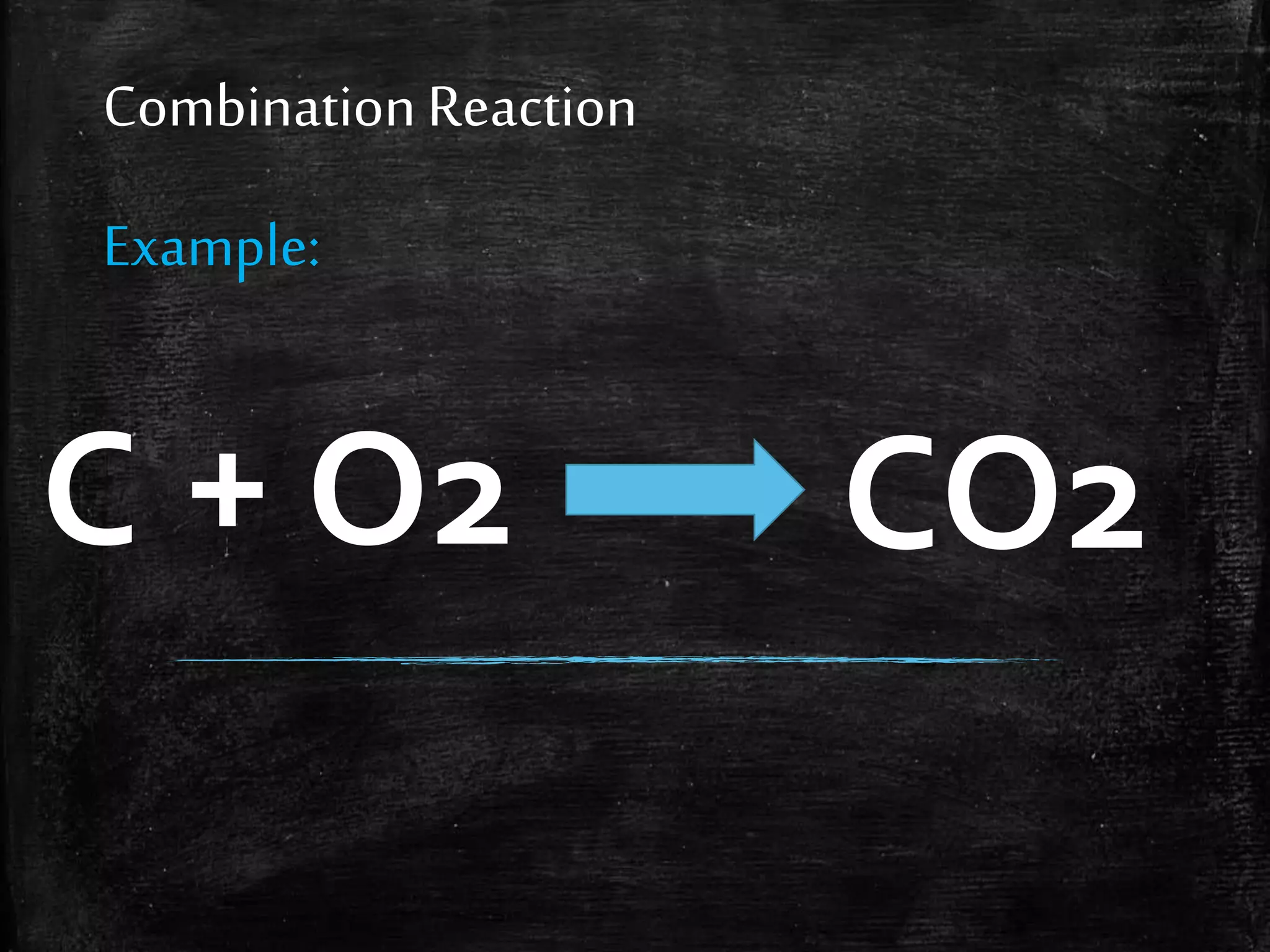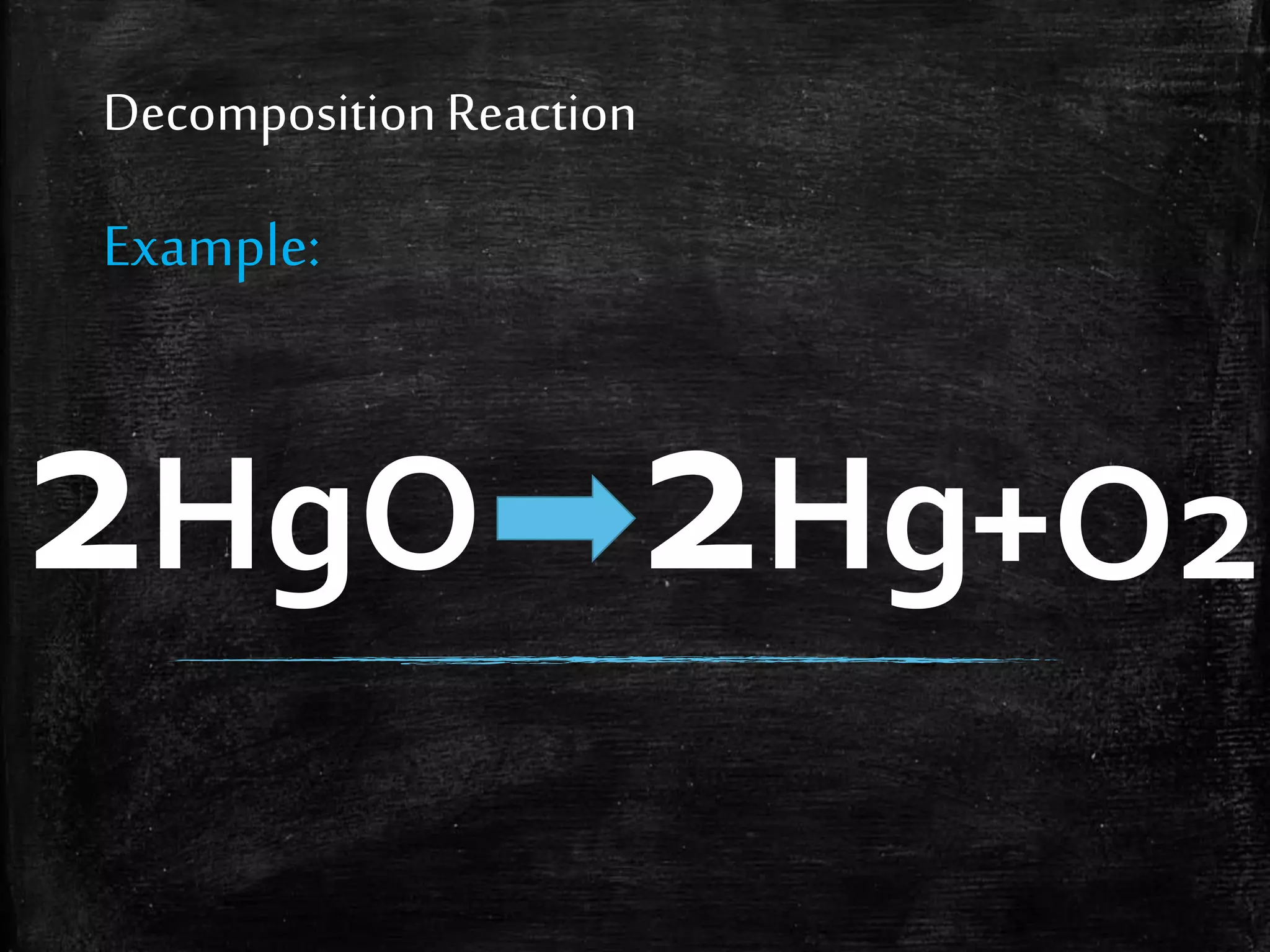This document contains notes from a chemistry laboratory class. It includes the chemical formulas for various compounds tested in a quiz, as well as sections on different types of chemical reactions like combination, decomposition, single displacement and double displacement reactions. Examples are provided for each type of reaction. Safety precautions for the laboratory are emphasized.