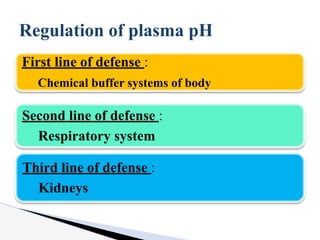
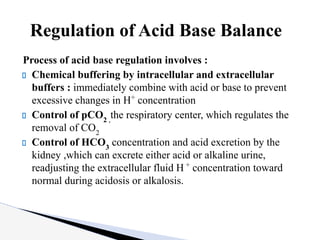
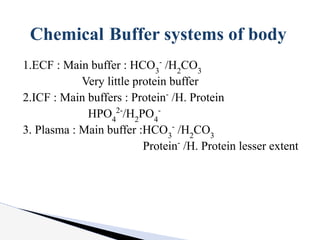
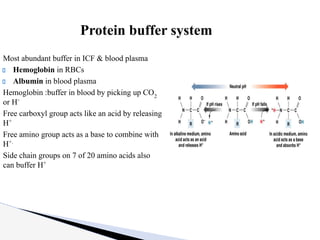
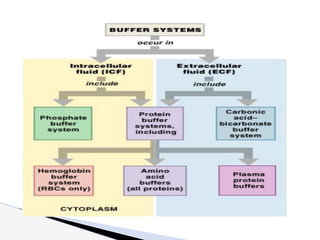
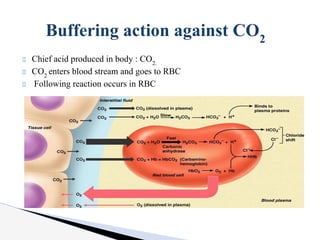
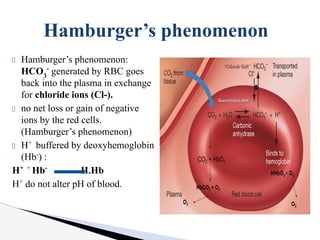
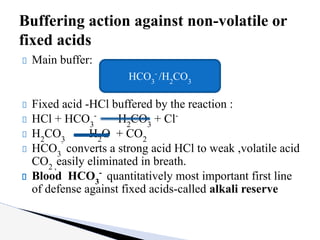
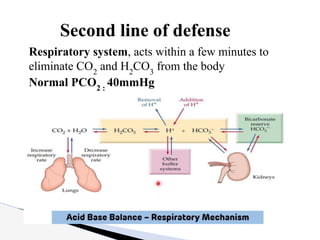
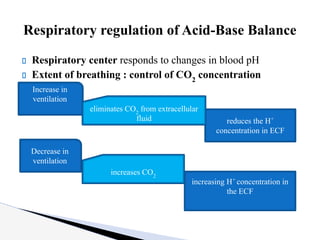
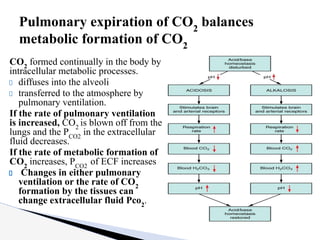
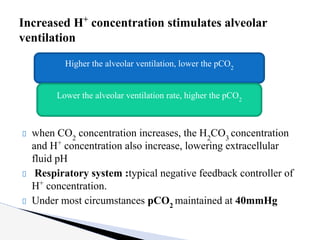
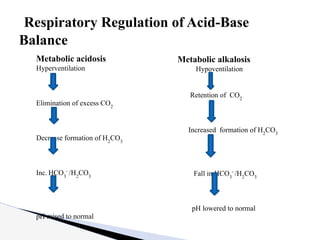
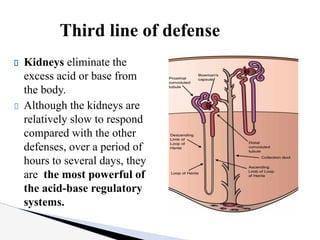
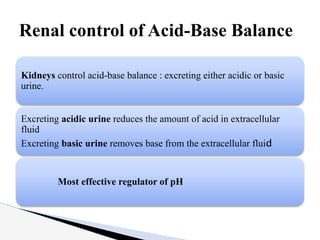
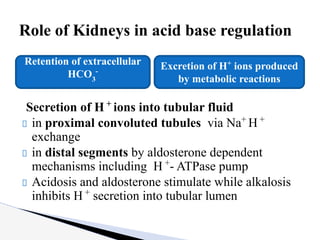
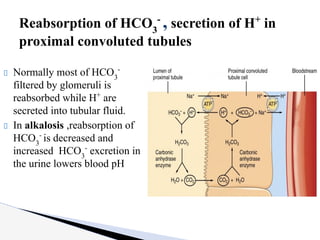
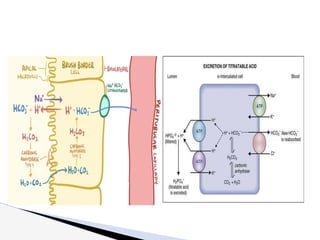
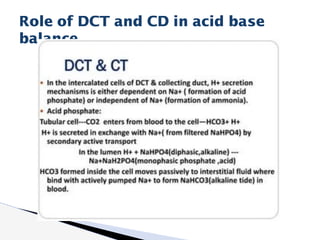
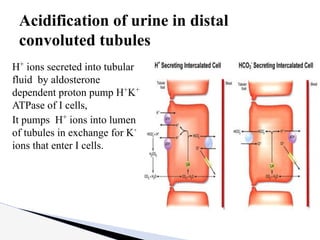
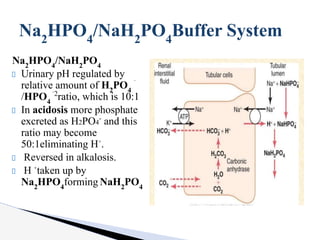
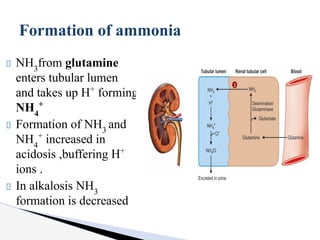
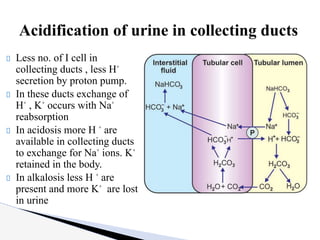
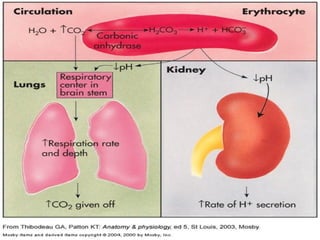
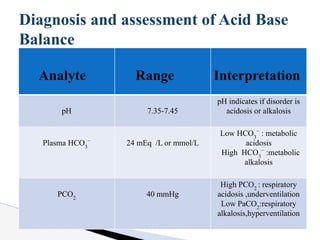
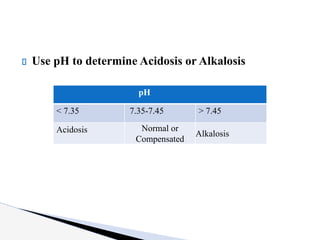
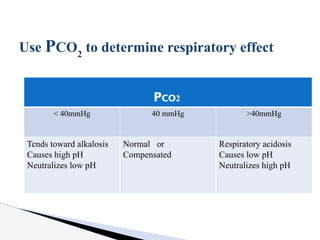
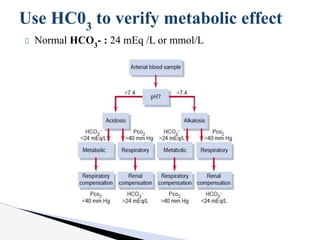
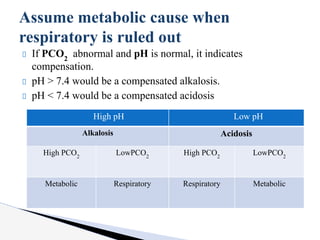
The document discusses the concept of acid-base balance, detailing its importance in metabolic and respiratory disorders through signs, symptoms, and arterial blood gas (ABG) findings. It outlines the roles of chemical buffers, the respiratory system, and kidneys in maintaining this balance, including mechanisms of acidosis and alkalosis. The text emphasizes the diagnostic interpretation of ABG results, indicating conditions such as metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis based on pH, pCO2, and bicarbonate levels.