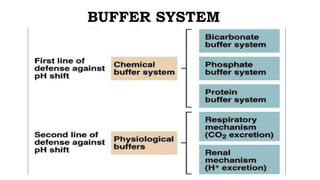
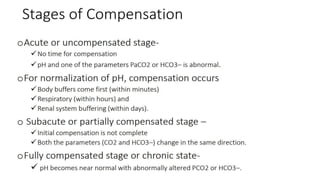
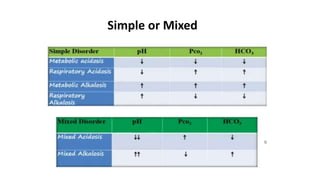
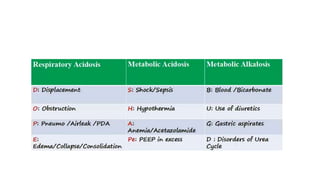
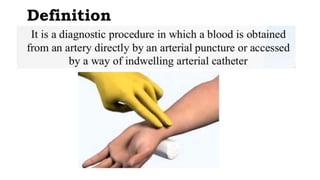
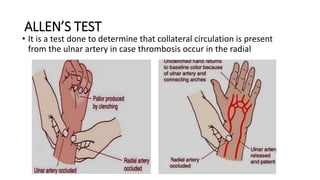
This document provides information on arterial blood gas (ABG) interpretation for medical professionals and trainees. It discusses the indications, equipment, and procedures for collecting an ABG sample. Key steps in the collection process include preparing the patient, selecting an appropriate artery site, and applying pressure after puncture. The document then covers interpreting ABG results through a stepwise approach considering pH, acid-base balance, compensation, oxygenation status, and identifying simple vs mixed disorders. Common abnormal values and their implications are presented. Examples of interpreted ABG reports are provided to demonstrate the evaluation process.














![Henderson-Hasselbalch Equation
Correlates metabolic & respiratoryregulations
HCO -
pH = pK + log ----------------
.03 x [PaCO2]
Simplified
HCO3 -
pH ~ ---------
PaCO2](https://image.slidesharecdn.com/abgmaster-230124194859-3aeb9f2e/85/ABG-INTERPRETATION-pptx-18-320.jpg)