The document outlines the fundamentals of acid-base balance, detailing blood pH, buffering systems, and types of acid-base disorders including acidosis and alkalosis. It explains the roles of blood components, the anion gap, arterial blood gases, and compensation mechanisms within the body. The document also describes common causes of metabolic and respiratory acid-base imbalances and methodical approaches to analyzing arterial blood gas results.


![ Normal arterial blood pH range from 7.35-7.45.
Blood pH inversely related to [H+], the more [H+] the lower the pH
Blood pH is maintained by the balance between acid production in the
body and the function of the buffering systems, kidneys & lung.
Buffering systems inculde: bicarbonate, phosphate, negative charged
proteins, ammonia (any – charged molecule act as a buffering molecule
that bind’s H+)
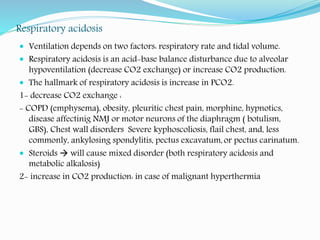
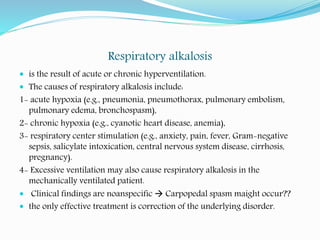
CO2 is considered acidic, we lose it by ventilation, (hyper ventilation =
more CO2 washout, hypoventilation = more CO2 retention in the body)
The kidneys also control acid-base balance, through absorption or
secretion of HCO3- & H+.](https://image.slidesharecdn.com/acid-base-190327121128/85/Acid-base-disorders-3-320.jpg)

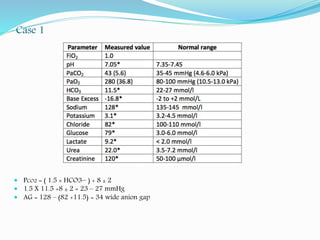
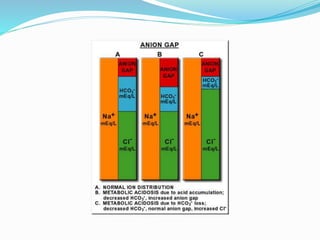
![Anion gap (AG)
Anion gap = [Na+] – ([HCO3-] + [Cl-])
AG represents the anions “other than Cl- and HCO3
-” that are necessary to
counterbalance Na+ electrically
Normal anion gap is 12 ± 2, any increase in AG > 14 is called wide anion
gap.
AG is only important in metabolic acidosis.
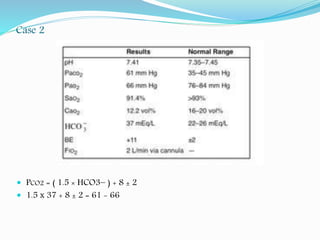
Arterial blood gases ABG
• Arterial blood gases include the measurement of arterial PaCO2, PaO2,
blood pH, [HCO3-], O2 saturation “O sat”.
• Mixed venous blood gases include the measurement of PvCO2, PvO2,
venous blood pH, [HCO3-], O2 saturation](https://image.slidesharecdn.com/acid-base-190327121128/85/Acid-base-disorders-6-320.jpg)




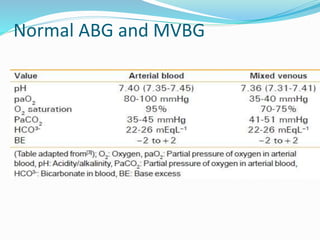
![How to read ABG
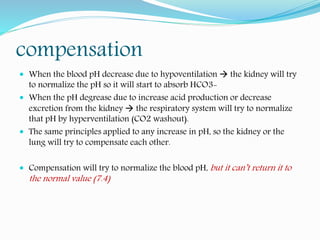
Check the pH 7.4 normal, (>7.4 alkalosis), (<7.4 acidosis)
Check [HCO3-]: is it directly related to pH or not?
- yes metabolic
no respiratory
normal check pCO2 if normal then normal ABG
is there appropriate compensation or not?
- yes pure metabolic or respiratory cause
no mixed condition (both respiratory and metabolic problem together)
if there is metabolic acidosis check the AG (wide or normal)](https://image.slidesharecdn.com/acid-base-190327121128/85/Acid-base-disorders-15-320.jpg)