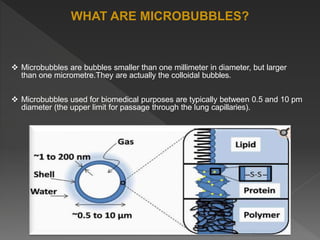
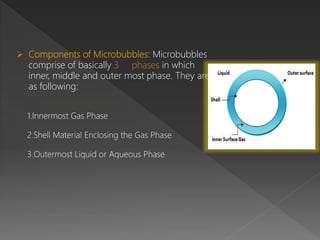
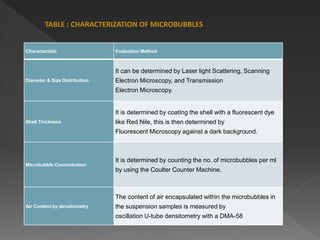
Microbubbles are bubbles smaller than one millimeter in diameter that have applications in industry, life sciences, and medicine. They consist of a gas core surrounded by a shell. Microbubbles are used as contrast agents in ultrasound imaging due to differences in density and compressibility compared to tissues. They can also be used for drug and gene delivery by incorporating drugs or genes into the shell or gas core. When exposed to ultrasound, microbubbles burst and release their contents locally for targeted therapy without affecting other body systems. Commonly used microbubble formulations include Albunex, Echovist, and SonoVue.
![MICROBUBBLE
Presentation By
SHAH ABDUL BARI
Guided By
DR.QAZI MAJAZ SIR
M.Pharm First Year
(QA SEM 1)
[Roll No. 08]
SEAT NO: 517628
Jamia Islamia Isha-atul Uloom’s
Ali-Allana College of Pharmacy
Akkalkuwa, Dist. Nandurbar.](https://image.slidesharecdn.com/abdulbariprojectautosaved-210727135139/85/A-project-on-microbubble-1-320.jpg)